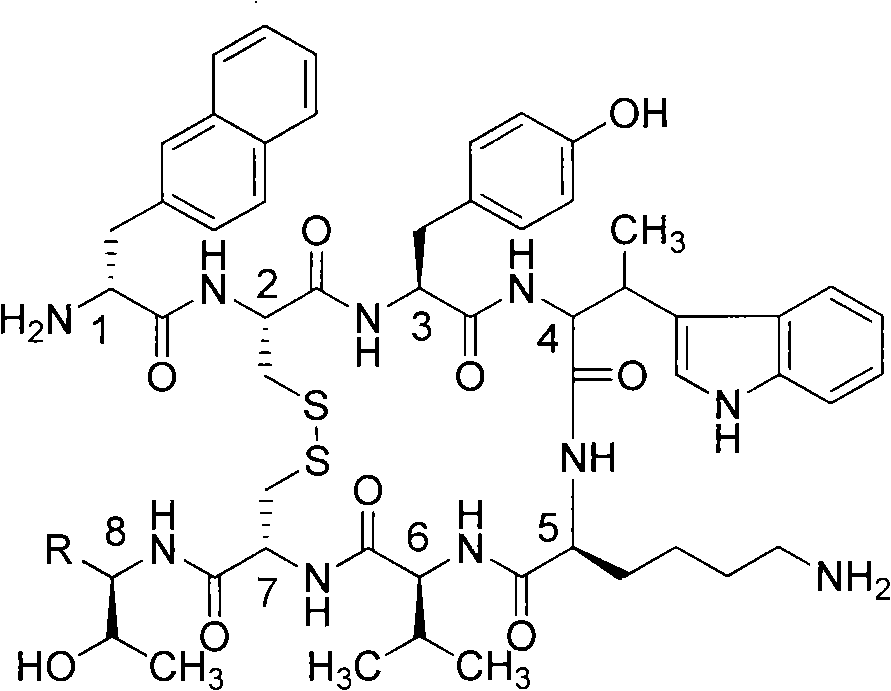
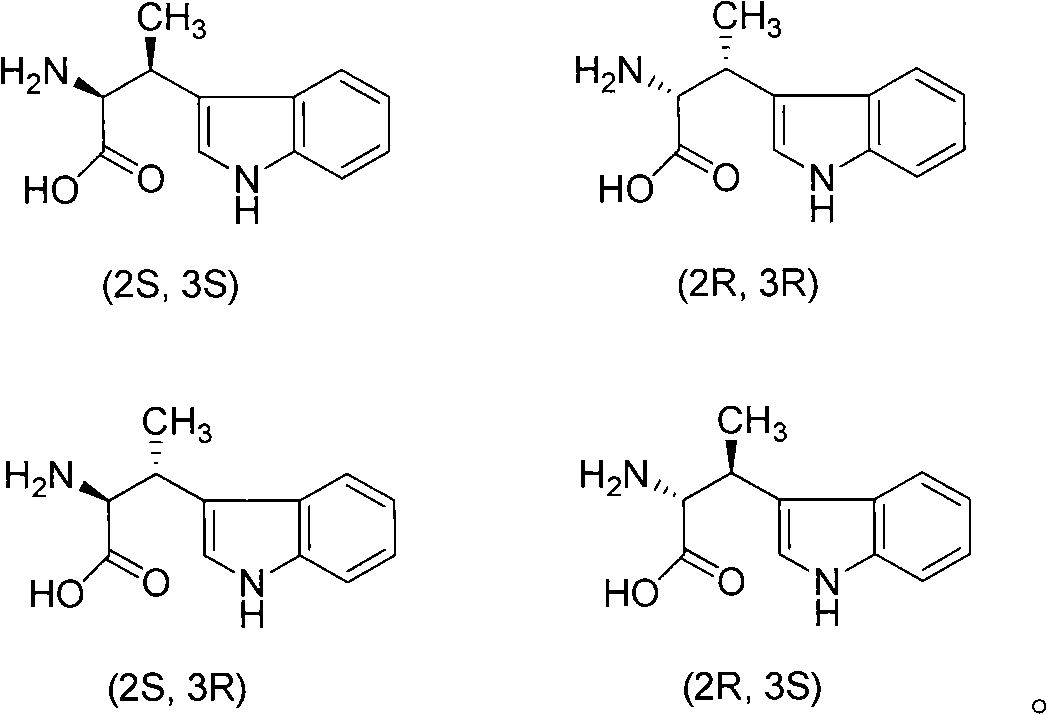
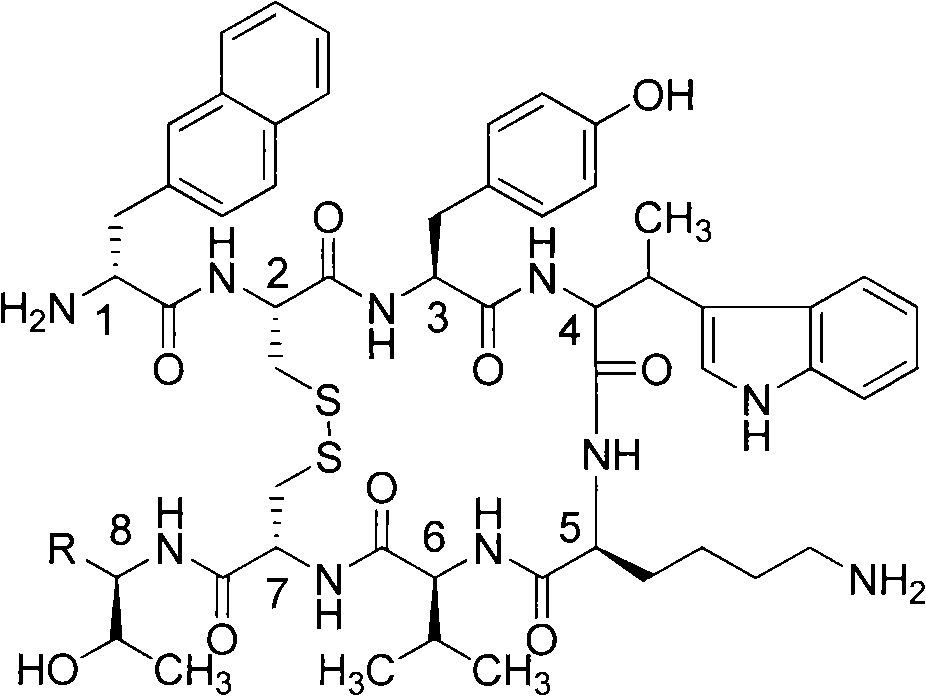
Growth inhibition hormone peptide derivatives and preparation thereof
A technology of peptide derivatives and growth inhibition, applied in the field of anti-tumor drugs, can solve the problems of lanreotide, such as difficult to distinguish affinity, unclear targeting, and clinical application limitations, so as to reduce binding, increase curative effect, and reduce toxic and side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 (R=COOH, cyclic polypeptide synthesis method A):
[0029] 2-Chloro-trityl resin (1 gram, degree of substitution: 0.7 mmol per gram), added dichloromethane (20 milliliters), swelled for two hours under the protection of nitrogen, filtered, and washed again with dichloromethane Twice, then add Fmoc-Thr(OtBu)-OH (2.1mmol), diisopropylethylamine (DIPEA, 8.4mmol), after two hours of reaction, filter, and wash twice with dichloromethane to obtain Fmoc -Thr(OtBu)-O-2-chloro-trityl resin. Next use the peptide synthesis steps as follows:
[0030] Use piperidine (25% N,N-dimethylformamide solution) to remove the Fmoc protecting group (20 minutes); the obtained H-Thr(OtBu)-O-2-chloro-trityl resin was used Dichloromethane and N,N-dimethylformamide were washed twice, each for two minutes; then the Fmoc-protected amino acid [here is Fmoc-Cys(Trt)-OH at the 7th position, 2.1 mmol] was added; HOBt (2.1 mmol); HBTU (2.1 mmol); diisopropylethylamine (DIPEA, 4.2 mmol) and N,N...
Embodiment 2
[0033] Example 2 (R=COOH, cyclic polypeptide synthesis method B):
[0034] NH 2 -trityl resin (1 gram, degree of substitution: 0.7 mmol per gram), adding dichloromethane (20 milliliters), swelling under the protection of nitrogen for two hours, filtering, washing twice with dichloromethane, Then add Fmoc-Thr(OtBu)-OH (2.1mmol); HOBt (2.1mmol); HBTU (2.1mmol); Diisopropylethylamine (DIPEA, 8.4mmol), react for two hours, filter, and dichloro After two more methane washes, Fmoc-Thr(OtBu)-NH-trityl resin was obtained. Next use the peptide synthesis steps as follows:
[0035] Use piperidine (25% N,N-dimethylformamide solution) to remove the Fmoc protecting group (20 minutes); the resulting H-Thr(OtBu)-NH-trityl resin was washed with dichloromethane and N, N-dimethylformamide was washed twice, each for two minutes; then added Fmoc-protected amino acid [here is the 7th Fmoc-Cys (Trt)-OH, 2.1mmol]; HOBt (2.1mmol ); HBTU (2.1 mmol); Diisopropylethylamine (DIPEA, 4.2 mmol) and N, N ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com