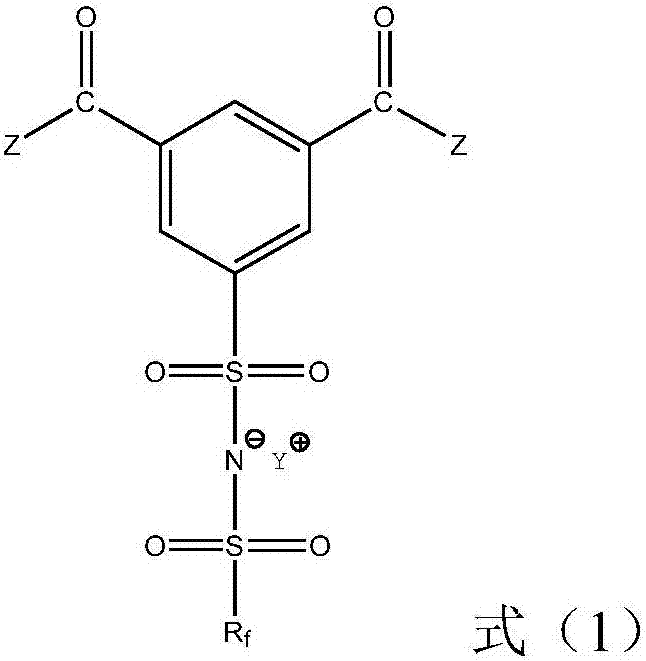
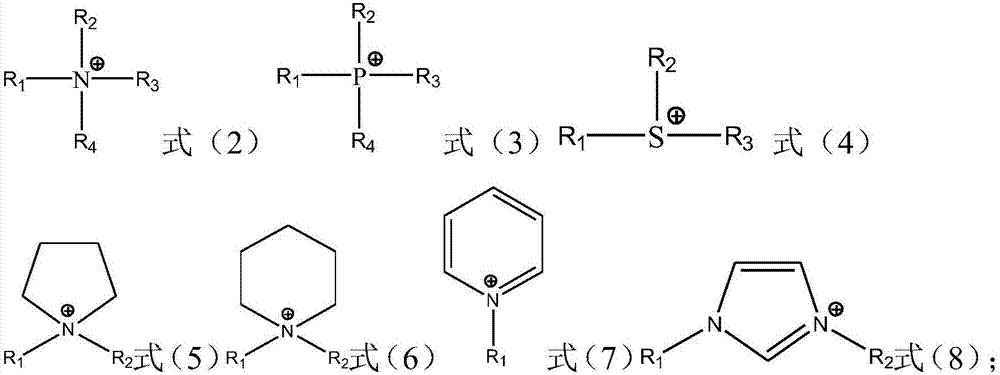
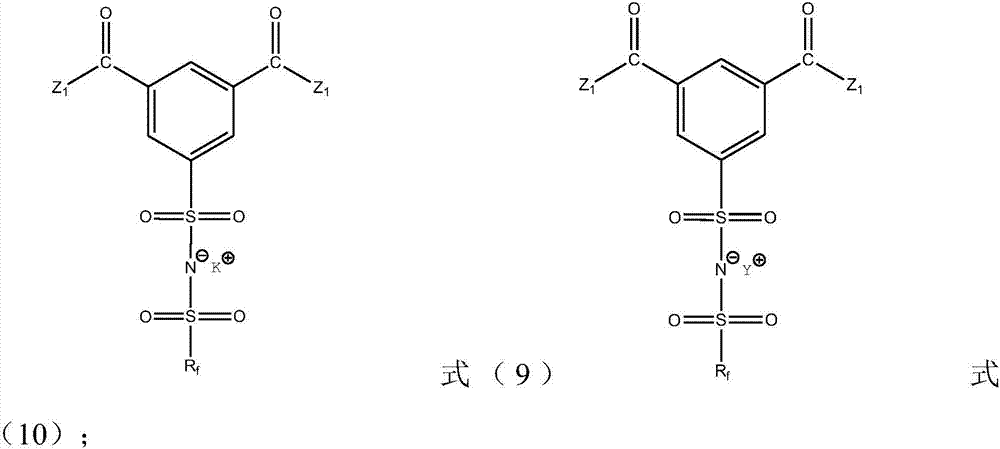
Ionic liquid compound, preparation method thereof, ionic liquid polymer, use of ionic liquid polymer and polymer solid electrolyte containing ionic liquid polymer
A technology of ionic liquids and compounds, which can be used in the preparation of hybrid capacitor electrolytes, capacitor electrolytes/absorbents, sulfonamides, etc., and can solve the problems of slow ion migration and low conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] Optionally, the preparation method of the compound shown in formula (9) comprises: making the compound shown in formula (12) contact with a halogenating reagent under halogenation reaction conditions:
[0064]
[0065] Among them, R f for C h f 2h+1 , h is an integer of 0-10.
[0066] Alternatively, the halogenation reagent can be selected from PX 5 and / or POX 3 , X is at least one of Cl, Br and I; the molar ratio of the compound represented by formula (12) to the halogenating agent can be 1:(2-6).
[0067] Optionally, the conditions of the halogenation reaction may be as follows: the reaction temperature is 0-200°C, the reaction time is 1-24h, and the solvent is at least one of dichloromethane, chloroform, acetonitrile, nitromethane and acetone.
[0068] Alternatively, the preparation method of the compound shown in formula (12) comprises: make the compound shown in formula (13) contact with alkali under neutralization reaction condition:
[0069]
[0070] ...
Embodiment 1
[0160] This example is used to illustrate the preparation method of the ionic liquid compound of the present disclosure.
[0161]
[0162]
[0163] 2.0467g (10mmol) of 3,5-dimethylbenzenesulfonyl chloride was reacted with 0.5109g (30mmol) of liquid ammonia at -35°C for 12h to obtain compound 1a (1.6672g, yield 90%); 1 H NMR (400MHz, CDCl 3 , ppm), δ=7.54(s, 2×1H), 6.90(s, 1H), 2.35(s, 2×3H), 2.0(s, 2H);
[0164] 1.8524g (10mmol) of compound 1a was reacted with 2.3794g (20mmol) of thionyl chloride and 1.2817g (11mmol) of chlorosulfonic acid at 100°C for 12h to obtain compound 1b (2.5538g, yield 90%); 1 H NMR (400MHz, CDCl 3 , ppm), δ=7.54(s, 2×1H), 6.90(s, 1H), 2.35(s, 2×3H), 2.0(s, 1H);
[0165] Take 2.8375g (10mmol) of compound 1b and 2.1451g (12mmol) of SbF 3 Reacted at 60°C for 12h to obtain compound 1c (2.4057g, yield 90%); 1 H NMR (400MHz, CDCl 3 , ppm), δ=7.54(s, 2×1H), 6.90(s, 1H), 2.35(s, 2×3H), 2.0(s, 1H);
[0166] Take 2.6730g (10mmol) of compound 1c and...
Embodiment 2
[0171]
[0172] Take 1.8718g (10mmol) of potassium trifluoromethanesulfonamide monohydrogen and 2.2514g (11mmol) of 3,5-dimethylbenzenesulfonyl chloride to react at 80°C for 12h to obtain compound 2a (2.8558g, yield 90%) ; 1 H NMR (400MHz, CDCl 3 , ppm), δ=7.54(s, 2×1H), 6.90(s, 1H), 2.35(s, 2×3H), 2.0(s, 1H);
[0173] Take 3.1731g (10mmol) of compound 2a and 3.7927g (24mmol) KMnO 4 Reacted at 100°C for 12h to obtain compound 2b (3.3954g, yield 90%); 1 H NMR (400MHz, CDCl 3 , ppm), δ=11(s, 2×1H), 9.14(s, 2×1H), 9.0(s, 1H), 2.0(s, 1H);
[0174] Take 3.7727g (10mmol) of compound 2b and 5.5284g (40mmol) K 2 CO 3 Reacted at 25°C for 2h to obtain compound 2c (4.9154g, yield 100%); 1 H NMR (400MHz, CDCl 3 , ppm), δ=8.76(s, 2×1H), 8.4(s, 1H);
[0175] Take 4.9154g (10mmol) of compound 2c and 4.1648g (20mmol) of PCl 5 Reacted at 60°C for 12h to obtain compound 2d (4.0703g, yield 90%); 1 H NMR (400MHz, CDCl 3 , ppm), δ=9.11(s, 2×1H), 8.9(s, 1H);
[0176] 4.5225g (10mmol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Initial decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com