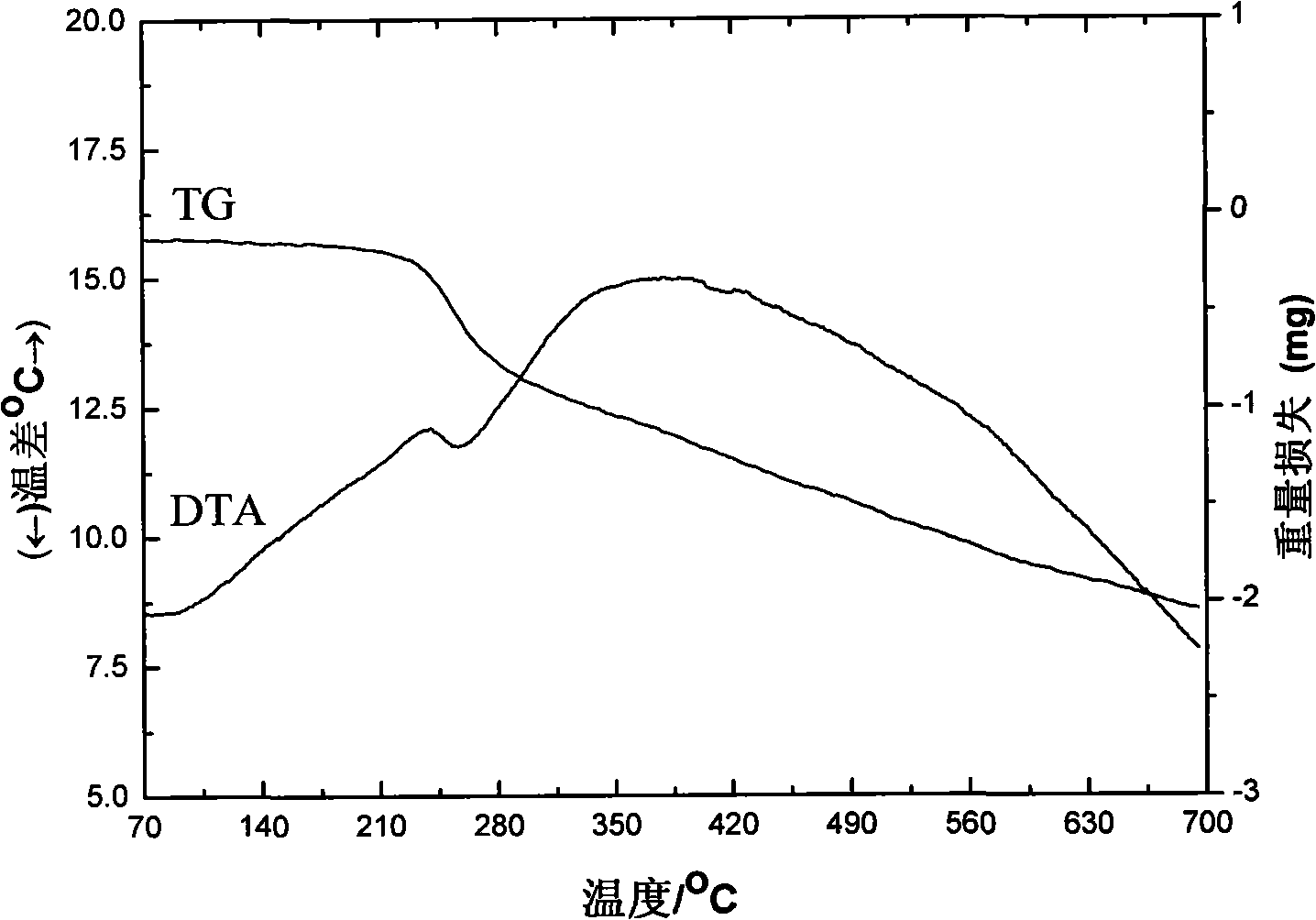
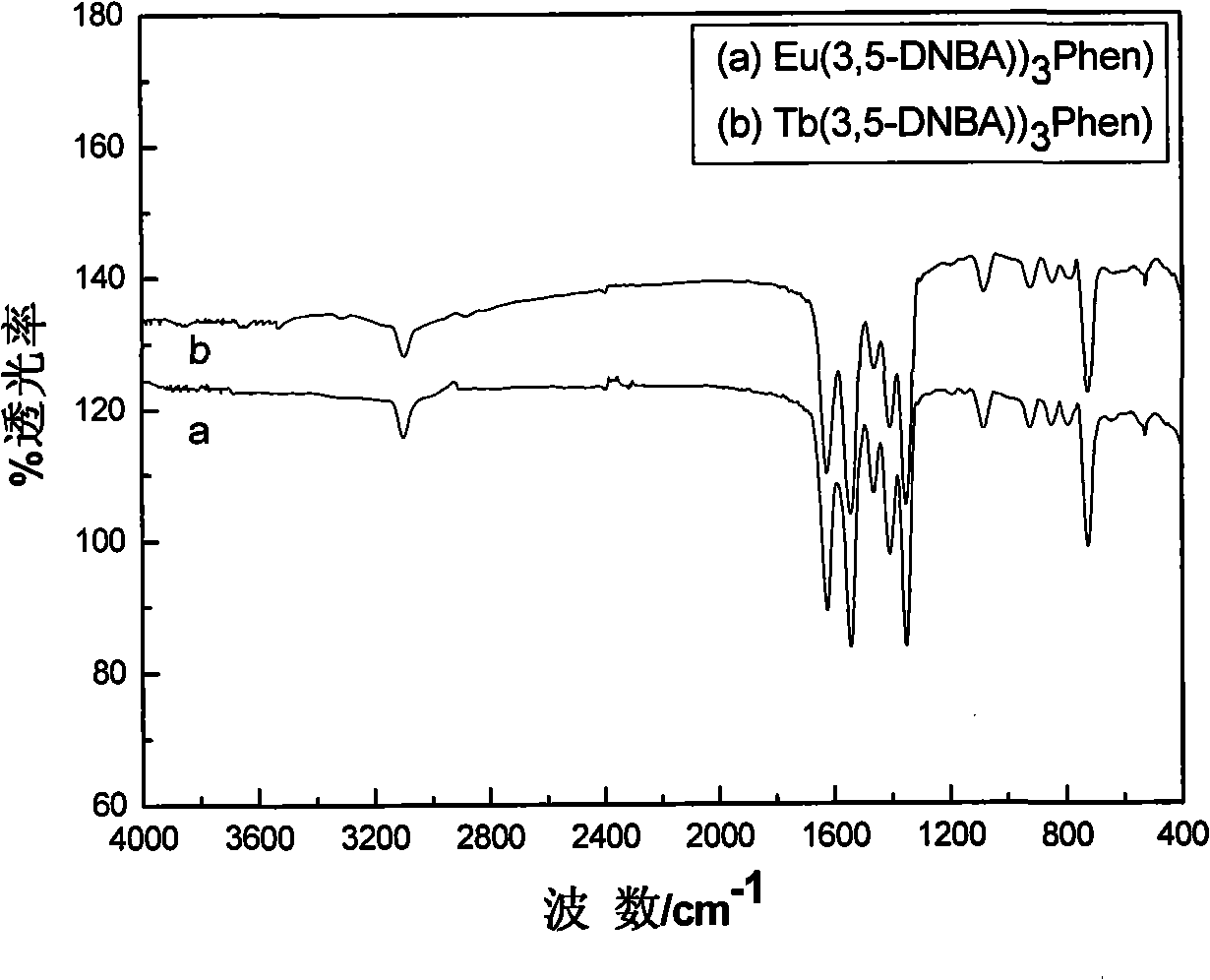
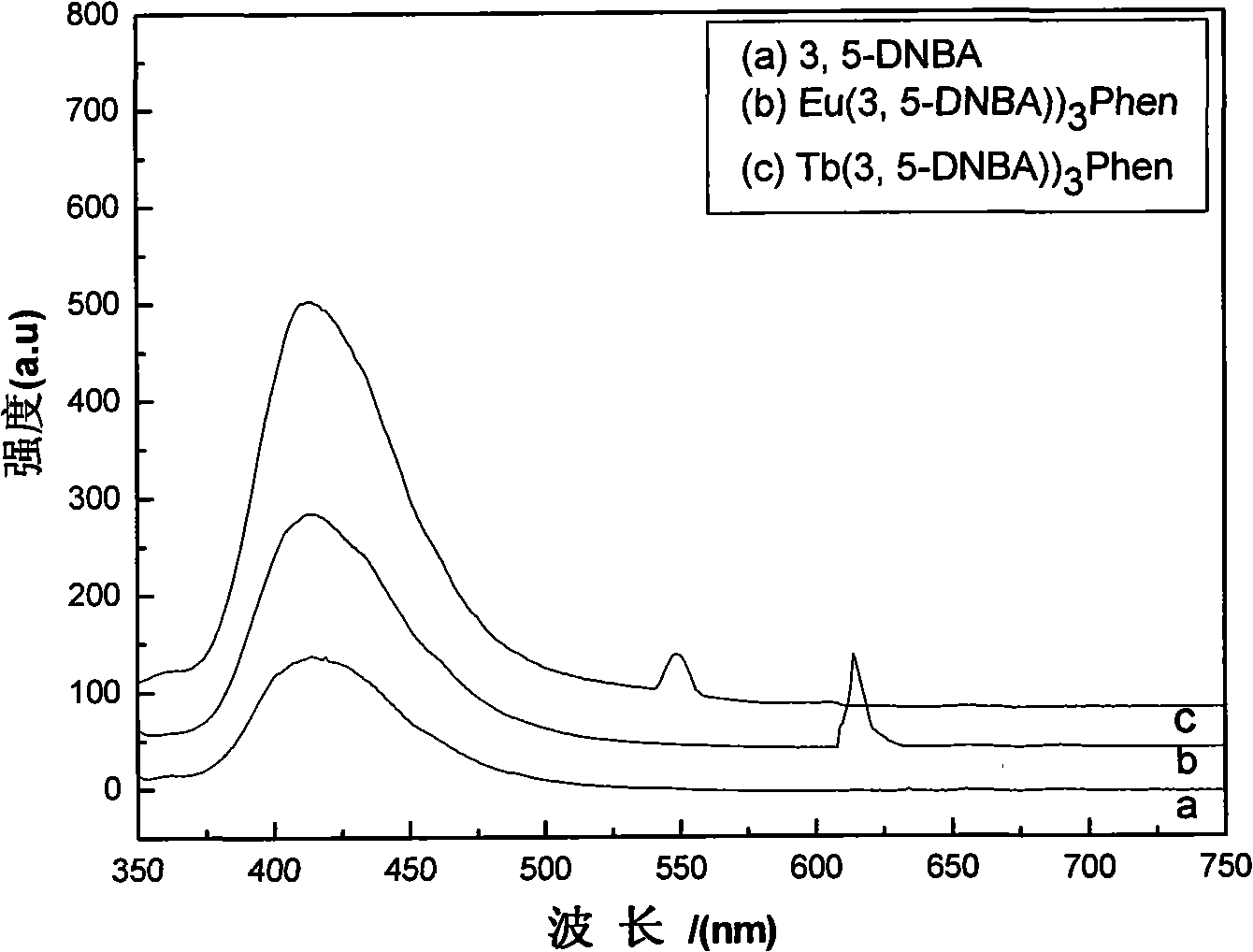
Rare earth aromatic carboxylic acid organic complex and preparation thereof
A technology of organic complexes and aromatic carboxylic acids, which is applied in the field of rare earth aromatic carboxylic acid complexes and their synthesis, can solve the problems of narrow application range, single luminous color of luminescent complexes, poor solubility of rare earth organic complexes, etc., and achieve Good stability and luminous performance, simple and easy process route, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A Press RE(3,5-DNBA) 3 Phen expression theoretical measurement, respectively take 0.1mol.L -1 EuCl 3 Add 0.1mol.L of ethanol solution 10ml under stirring -1 10ml of 1,10-Phen ethanol solution;
[0028] B. Then heat in a 60°C water bath, and slowly drop in 0.1mol.L under constant stirring -1 3,5-DNBA ethanol solution 30ml;
[0029] C. Adjust the pH value to 6.5 with triethylamine to produce a white precipitate. Let the above reactant stand at room temperature for 24 hours, let it mature, filter it with suction, and wash the precipitate with 95% ethanol until there is no Cl - , dried in a 70°C oven to obtain a white solid rare earth organic complex Eu(3,5-DNBA) 3 Phen.
[0030] In the rare earth organic complex Eu(3,5-DNBA) 3 Doped Nano TiO in Phen 2 , to improve the luminous intensity of rare earth organic complexes. The first is to prepare the light-emitting layer of the device, that is, the rare earth complex Eu(3,5-DNBA) 3 Phen, polymer PVK (polyvinylcarbaz...
Embodiment 2
[0032] A Press RE(3,5-DNBA) 3 Phen expression theoretical measurement, respectively take 0.2mol.L -1 TbCl 3 Ethanol solution 10ml, add 0.2mol.L under stirring -1 10ml of 1,10-Phen ethanol solution;
[0033] B. Heat to a constant temperature in a water bath at 62°C, and slowly add 0.2mol.L dropwise under constant stirring -1 m-NBA ethanol solution 30ml;
[0034] C. Adjust the pH value to 6.8 with triethylamine to produce a white precipitate. Let the above reactant stand at room temperature for 20 hours, let it mature, filter it with suction, and wash the precipitate with 95% ethanol until there is no Cl - , dried in an oven at 65°C to obtain the white solid rare earth organic complex Tb(3,5-DNBA) 3 Phen.
Embodiment 3
[0036] A Press RE(3,5-DNBA) 3 Phen expression theoretical measurement, respectively take 0.25mol.L -1 EuCl 3 Ethanol solution 10ml, add 0.25mol.L under stirring -1 10ml of 1,10-Phen ethanol solution;
[0037] B. Heat to a constant temperature in a water bath at 62°C, and slowly drop in 0.25mol.L under constant stirring -1 m-NBA ethanol solution 30ml;
[0038] B Use triethylamine to adjust the pH value to 7.0 to produce a white precipitate, let the above reactant stand at room temperature for 23 hours, let it mature, filter it with suction, and wash the precipitate with 95% ethanol until there is no Cl - , dried in an oven at 66°C to obtain a white solid rare earth organic complex Eu(3,5-DNBA) 3 Phen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com