Isoquinoline compounds, preparation method and use thereof
A technology of isoquinoline and compounds, applied in the field of isoquinoline compounds, can solve problems such as limited, lack of means for structural and functional research, and lack of specific drugs
Inactive Publication Date: 2008-11-26
SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
View PDF0 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, the current research on D5 receptors is still quite limited. On the one hand, people still lack the means to study the structure and function of this receptor. Generally, based on the similarity between D1 and D5 receptors, they use D1 receptors similar to D1 receptors. However, this method cannot effectively distinguish D1 and D5 receptors; on the other hand, the lack of D5 receptor-specific drugs also makes it more difficult to study the pharmacological effects and functions of D5 receptors
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
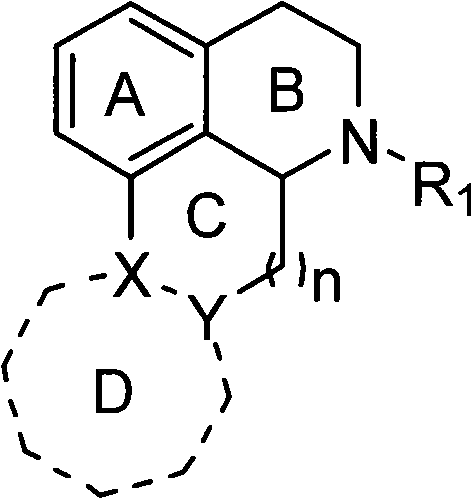
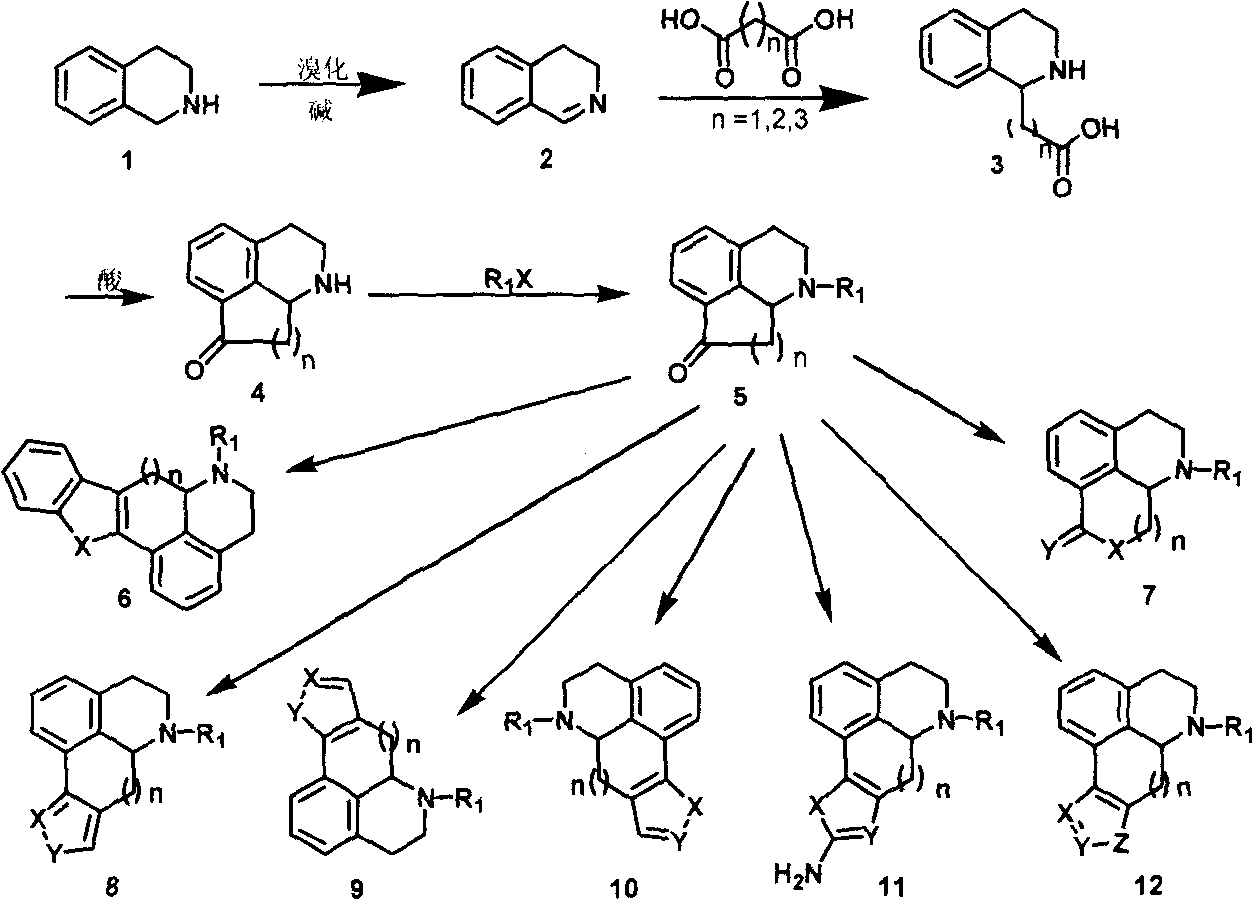
The invention discloses a novel isoquinoline compound, and a synthetic method thereof as well as an application of the compound, which is used as an agonist or an inhibitor of dopamine receptors in the medicines for treating Parkinson's disease, schizophrenia, tristimania, Tourette syndrome, attention-deficit hyperkinetic syndrome, pituitary tumor, etc. The constitutional formula of the compound is as follows: wherein, R1 is the hydrocarbyl with a H, C1-C10 linear chain or fork chain, heteroatom-substituted hydrocarbyl and aromatic or fatty heterocycle or non-heterocycle substituted alkyl; X is carbonyl, methylene and imidogen, and is more than C-OH or more than C=; Y is carboxide, methylene and imidogen, and is more than C= or more than C=N-OH; the compound can have no D cycle or the D cycle can be various heterocycles of substituted five-membered fat and substituted six-membered fat or substituted five-membered aromatic and six-membered aromatic; n is 0-3.
Description
technical field The invention relates to a class of isoquinoline compounds, a preparation method and uses thereof. This type of compound can be used as an agonist or antagonist of dopamine receptors, and can be used to treat neurological and mental diseases related to the brain, such as Parkinson's disease, schizophrenia, Tourette syndrome, attention deficit hyperactivity syndrome and pituitary tumors. Background technique Dopamine is the most abundant neurotransmitter in the mammalian brain. It has a catecholamine structure and its chemical name is 3,4-dihydroxyphenethylamine, 4-(2-ethylamino)benzene-1,2-diol, or 4-hydroxytyramine (abbreviated as DA). It is hydroxylated from L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA) by tyrosine hydroxylase in neurons, and then decarboxylated by aromatic decarboxylase to complete biosynthesis. Dopamine, as a message transmitter, can help nerve cells transmit nerve impulses generated by cells affected by external factors. Dopami...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D471/06C07D513/06C07D498/06C07D221/16A61K31/473A61K31/4738A61P25/00
Inventor 张翱刘致礼镇学初冯林音
Owner SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com