Application of TGF-beta1 inhibitory polypeptide on immune response modifier preparation
A technology of immune response and modulators, applied in the direction of allergic diseases, peptide/protein components, and diseases transmitted by vectors, and can solve problems that do not involve the use of immunomodulators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
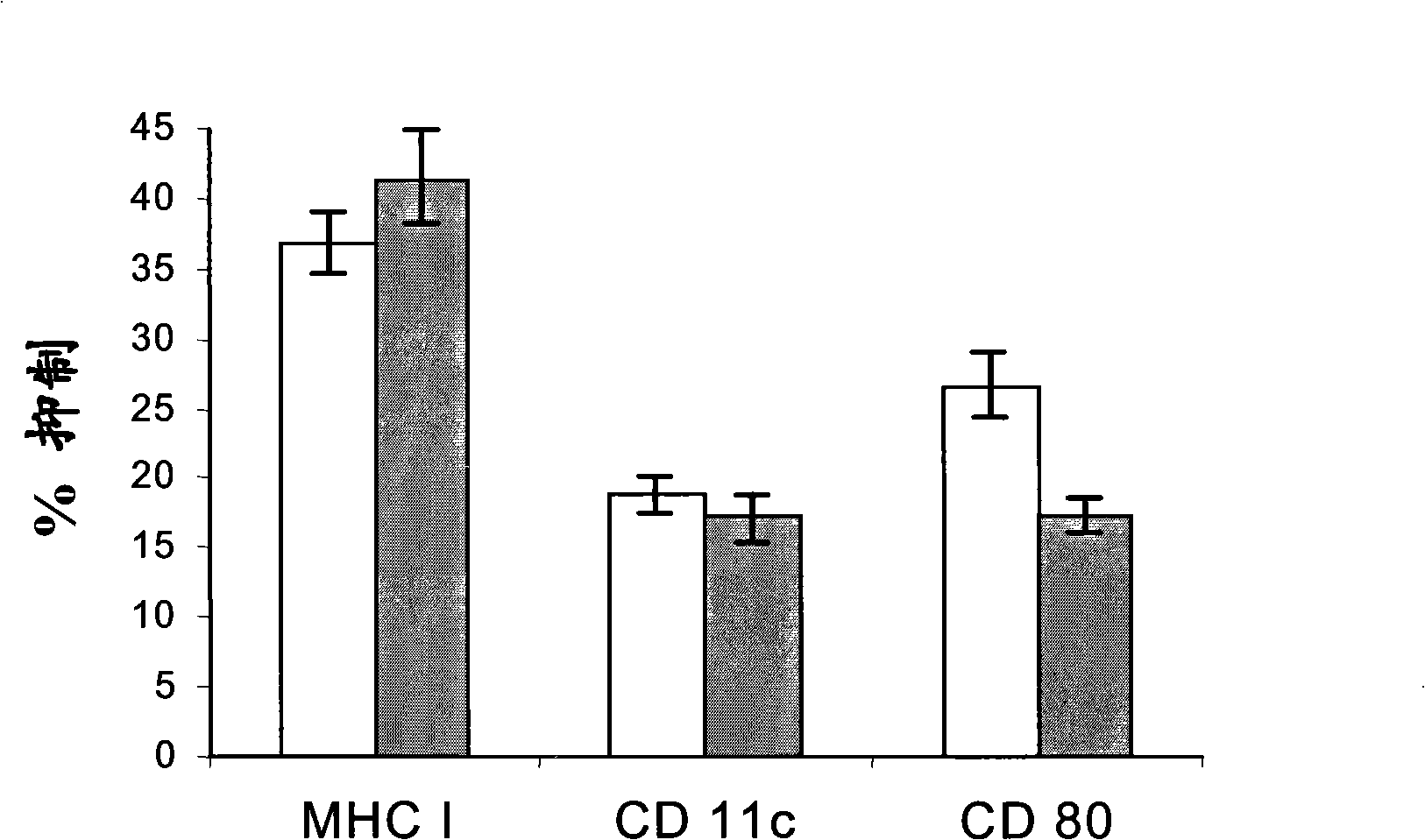
[0059] This example investigates the effect of the peptide p144 in a system in which exogenous TGF-β1 is used as an inducer of the differentiation of splenocyte populations into dendritic cells.
[0060] Isolation and culture of dendritic cells
[0061] After sacrifice of 8-week-old male C57 mice, their spleens were aseptically extracted and homogenized on plates with clean medium to generate cell suspensions. Centrifuge the cells at 1000 rpm for 5 minutes, and use 1 ml / spleen of ACK lysis solution (0.15M NH 4 Cl, 1 mM KHCO 3 , 0.1M EDTA sodium salt solution, pH 7.2-7.4) and resuspend the resulting cell pellet at 37°C for 1 min. Next, centrifuge the cells and use 10 ml of cold R10 medium (RPMI-1640, 10% FBS, glutamine, 2×10 -5 M 2-mercaptoethanol) to wash the cells to centrifuge and wash again. Finally, the cells were resuspended in 50 ml of R10 medium. Prepare a 6-well plate (Costar#3471), and use 1ml of R10 per well to treat them for 15 minutes at room temperature to d...
Embodiment 2
[0072] In vivo activity of recombinant adenovirus for mouse IL-12
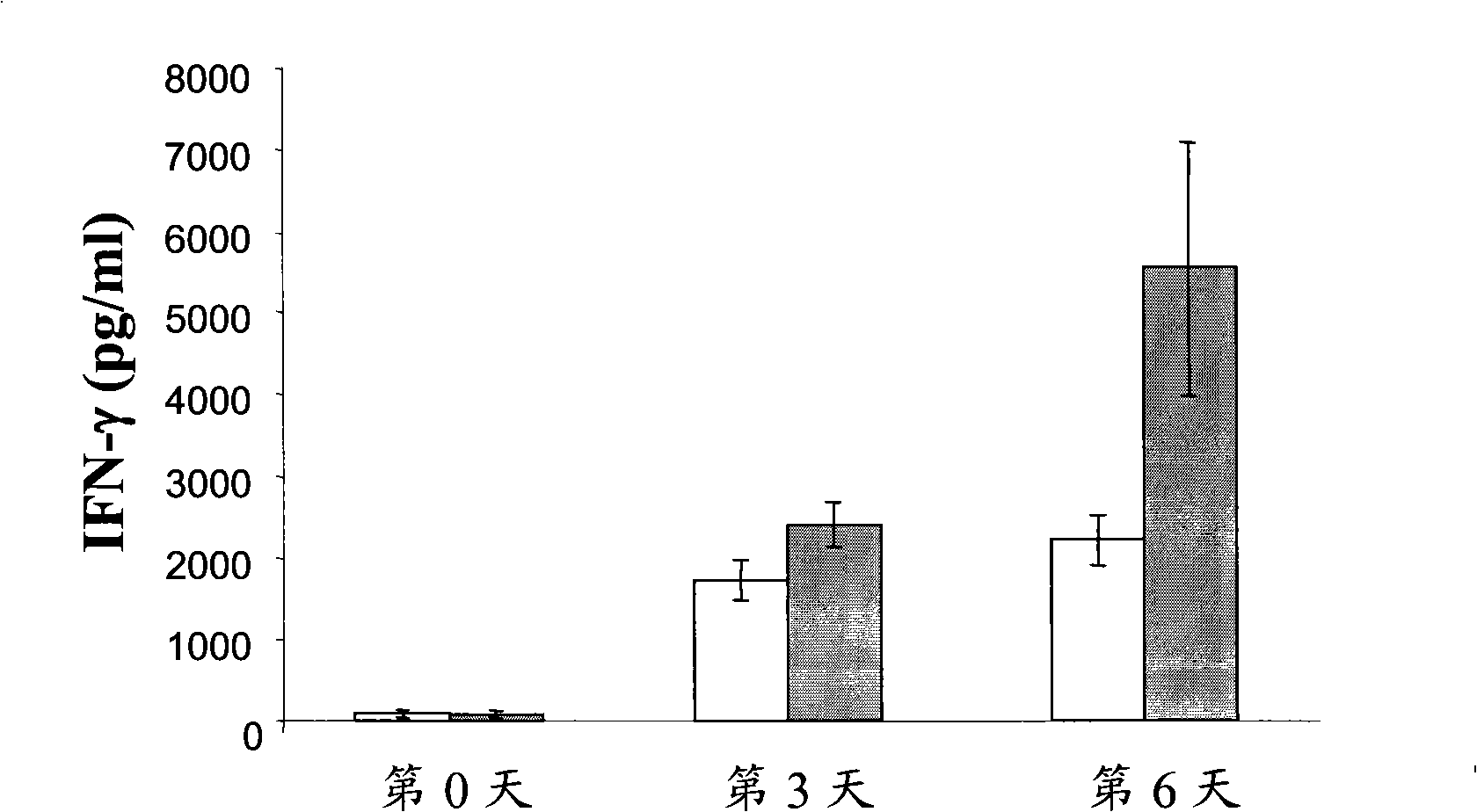
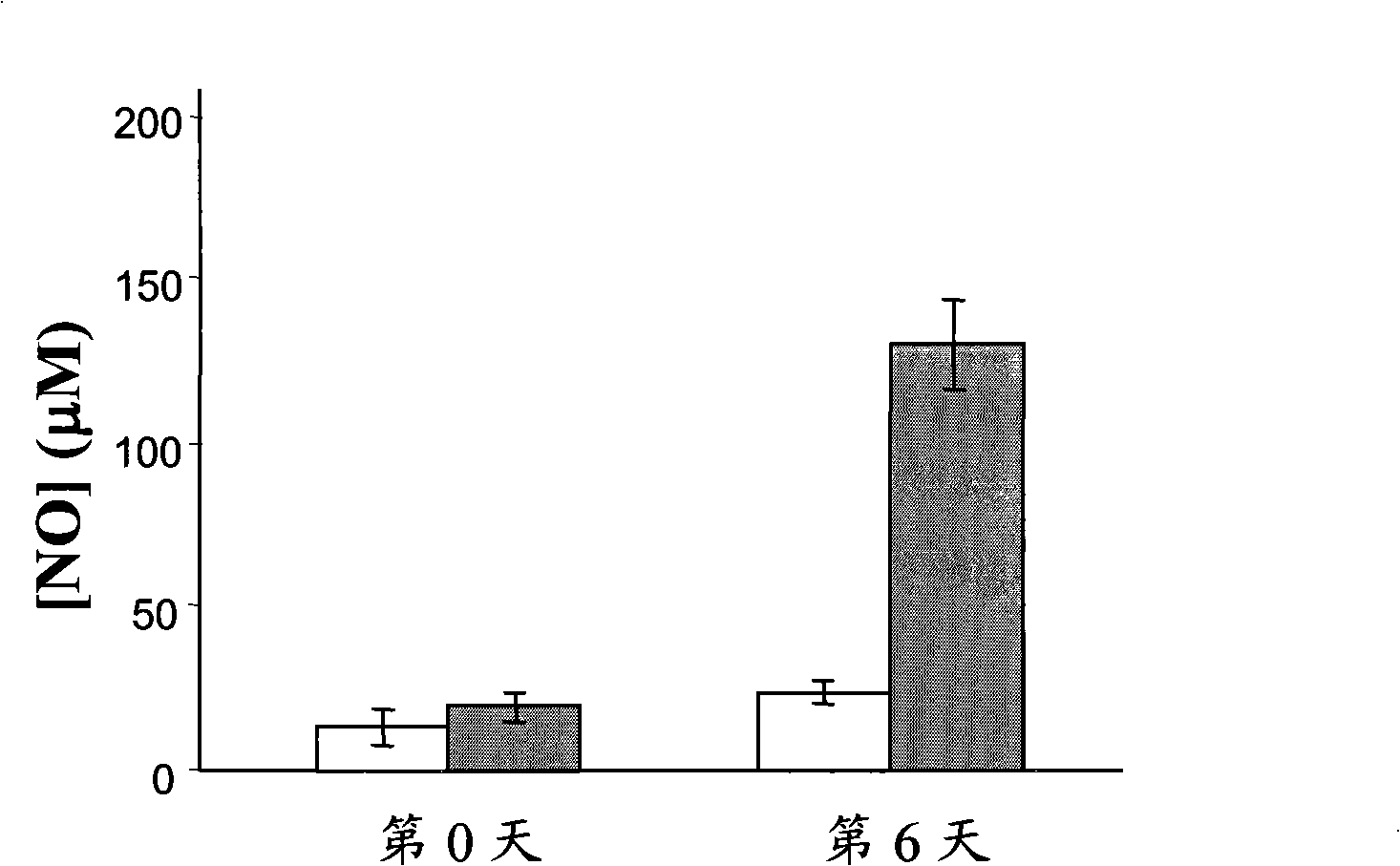
[0073] This example investigates the role of the peptide p144 in an in vivo system in which TGF-β1 acts as a putative antagonist of the cytokine induced in this model. Mouse IL-12 produced by expression of the transgene contained within the recombinant adenovirus induces an inflammatory state through the induction of a cascade of factors including IFN-γ and nitric oxide. TGF-β1 has been described as an inhibitor of the production and biological effects of IL-12, IFN-γ and NO (Pardoux C. et al., 1999; Schini V.B. et al., 1992). In this model, groups of 3 BALB / c mice were intraperitoneally administered 1×10 8 pfu of mouse RAd IL-12, the mice were 4 to 8 weeks old (Harlan), and the animals were allocated as follows:
[0074] Rad IL-12: The animals received 1 x 10 8 pfu of mouse RAd IL-12.
[0075] Rad IL-12+p144: the animals received the same treatment as the previous group, but they were given the peptide fo...
Embodiment 3
[0094] antibody induction
[0095] For the analysis of the immunomodulatory effect of peptide pl44 on humoral responses, 6- to 8-week-old female BALB / c mice (Harlan, Barcelona) were used. For the induction of specific antibodies, inactivated recombinant adenovirus (RAd-LacZ) was inoculated by heating in a water bath at 100° C. for 10 minutes.
[0096] Animal groups and treatments
[0097] Three mice per group were immunized with 200 μl of intraperitoneal mixture containing 1 × 10 8 pfu of inactivated RAd-LacZ adenovirus, physiological serum (PS) or peptide p144 with 50 μg, all as indicated in Table M4, emulsified in Freund's complete adjuvant (FCA) in a 1:1 volume ratio. Thirty days after the first immunization, the animals were immunized again with the same mixture but emulsified in Freund's incomplete adjuvant (FIA). Blood samples were taken from the retroorbital plexus on days 15 and 45 to quantify the production of anti-adenovirus antibodies by each animal.
[0098] To...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com