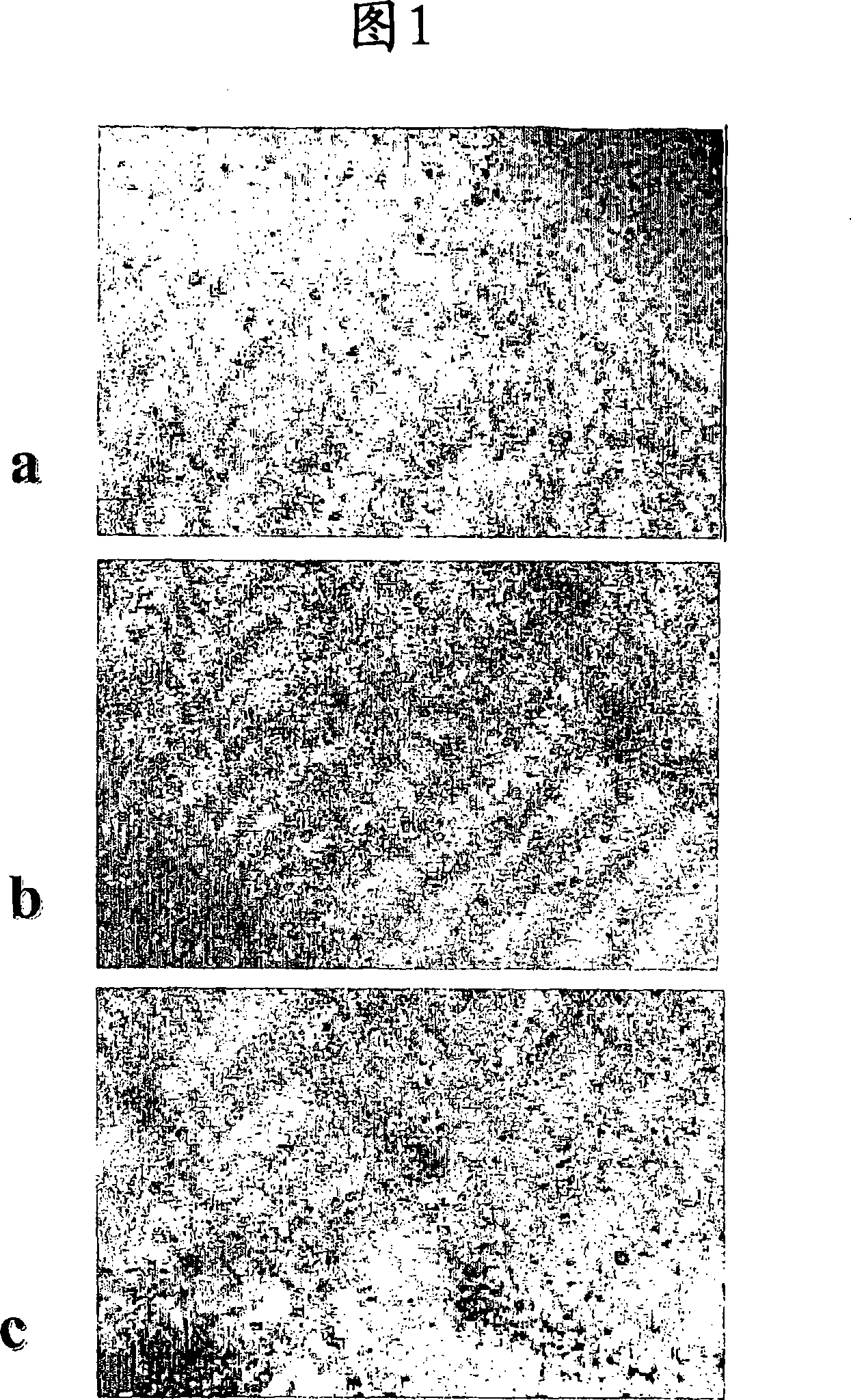
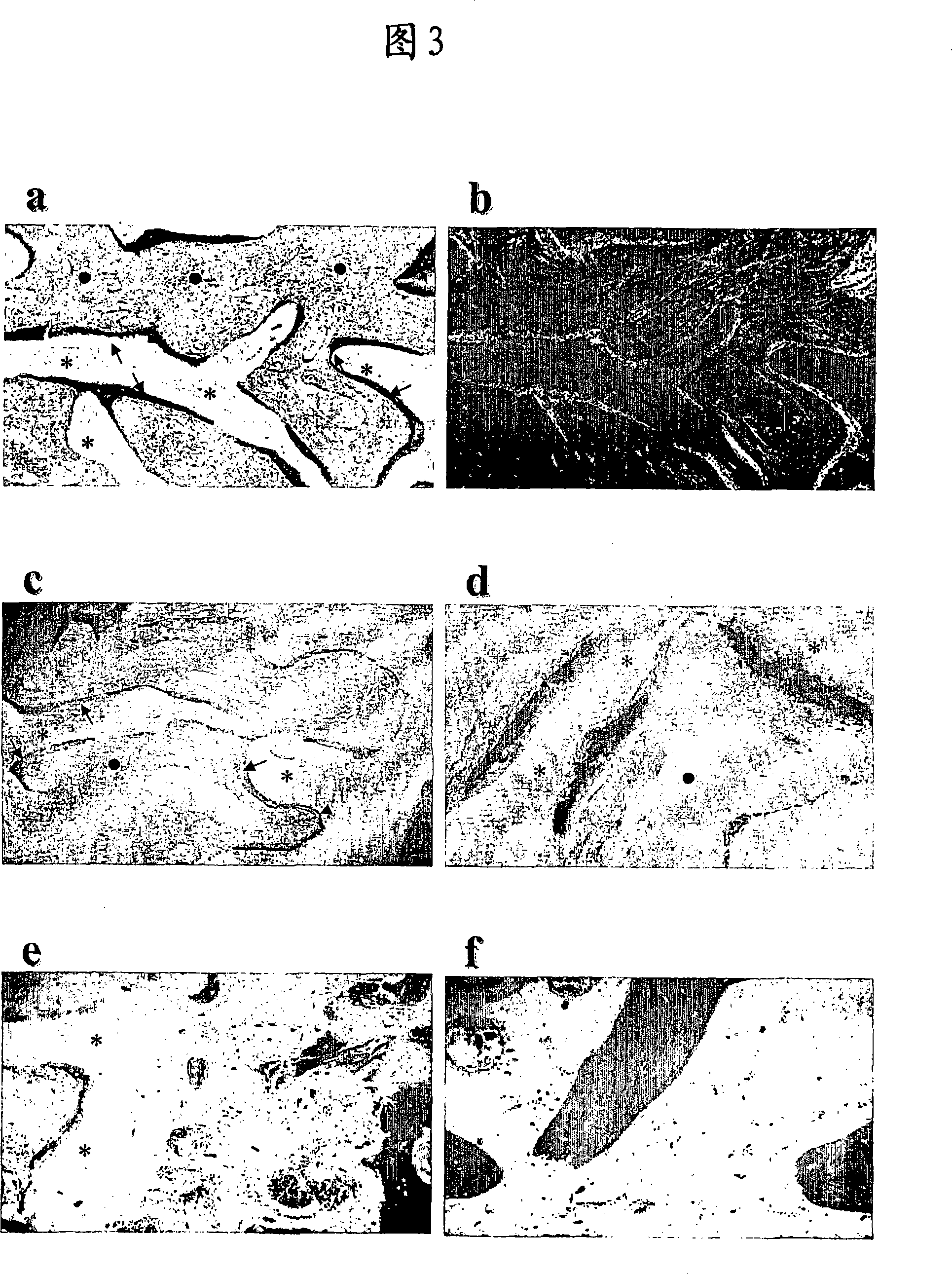
Cartilage and bone repair composition.
A technology of cartilage and bone formation, applied in the direction of drug combination, bone/connective tissue cells, microorganisms, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 - Design and acquisition of human fusion recombinant protein TGF-β1 (rhTGF-β1-CBD) with collagen I binding domain
[0034] The cDNA of TGF-β1 was obtained by RT-PCR using a proofreading-capable DNA-polymerase. The template used was total RNA, extracted from human osteosarcoma cells MG63 (ATCC reference: CRL-1427) by the method of Chomczynski and Sacchi (1987). Primers were designed according to the mRNA sequences published in Genbank with the help of software (Oligo v6.6). PCR products were cloned "in dull" in a maintenance plasmid (pBSK II, Stratagene). Once the gene has been cloned, it is tested by double-strand sequencing. Using these plasmids as templates, the region encoding the mature peptide was amplified by PCR with pfu-DNA-polymerase. The primers used contained the collagen binding domain (CBD) and restriction sites suitable for directional cloning in the transfer plasmid of the baculovirus expression system, and contained insect cell-specific sequ...
Embodiment 2
[0041] Example 2.-Obtaining and preparing human mesenchymal stem cells
[0042] Human mesenchymal stem cells were obtained from human bone marrow (BM) following surgical removal of the iliac crest from an informed donor. Bone marrow tissue was cultured in complete medium (-MEM, GIBCO , HD, US). Suspensions of individualized cells were obtained after successive dispersions through 18-, 20- and 22-gauge needles and finally filtered through a 20 micron Teflon sterile filter (Cell Strainer, Falcon, Lincoln Park, NJ) for tissue isolation Residues and cell buildup.
Embodiment 3
[0043] Example 3.- MSC Culture in Collagen Gel
[0044] The cell suspension obtained according to Example 2 was centrifuged at 100 g for 5 minutes. The centrate was resuspended in medium containing very low concentration of patient serum (HS, 0.5%).
[0045] Before the cells were placed in the wells of a 48-well culture plate, 100 mL of a human collagen I solution (Sigma-Aldrich), pH 7.4, at a concentration of 0.35 mg / mL, was placed at the bottom thereof. Mix 150 mL per well of a mixture of collagen I and TGF-β1 at a concentration of 1 mg / mL, and add 2 × 10 6 bone marrow cells on top. The culture plates were placed in a 37°C incubator for 15 minutes to allow the collagen, which was liquid before that time, to form a collagen gel. Then 10 mL of culture medium was placed in each well, also containing the same concentration of TGF-β1. Place the culture thus prepared in 95% air and 5% CO 2in an atmosphere of 37°C and 100% relative humidity. Every 3 days, the medium was chang...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com