Triazole derivatives
A technology of derivatives and drugs, applied in the field of triazole derivatives, can solve the problems of tissue damage, treatment-dependent activation and harmfulness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
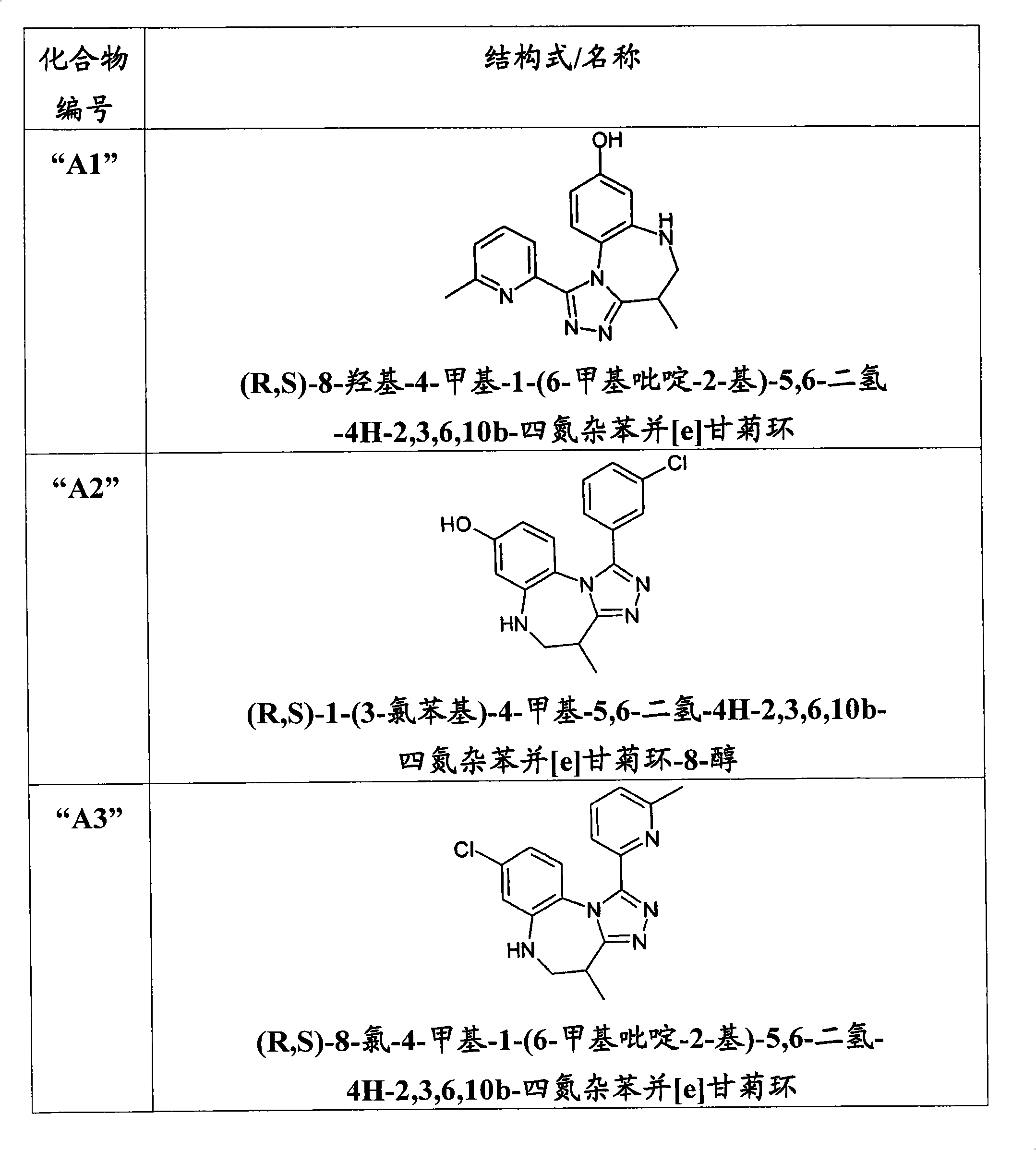
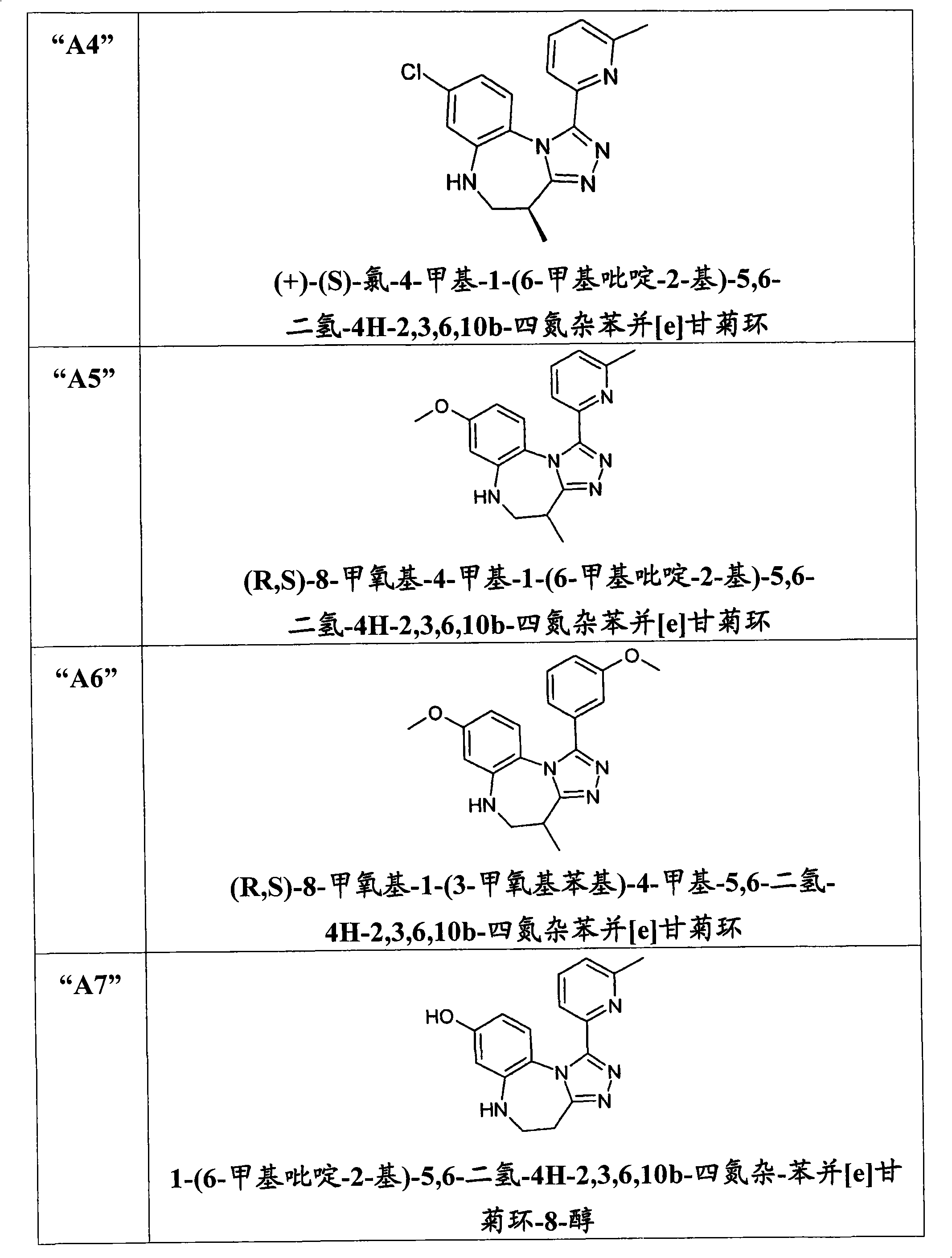
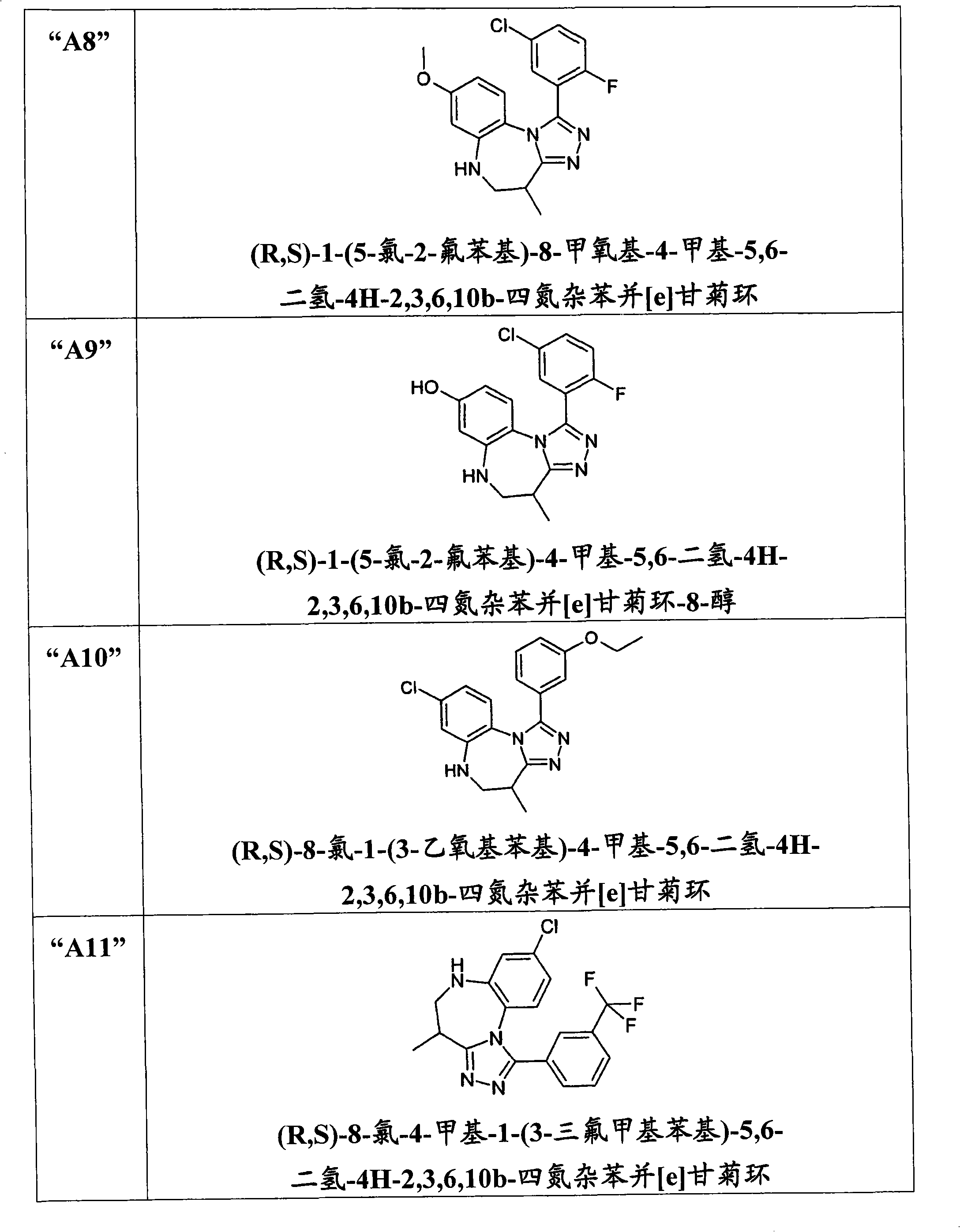
[0201] (R,S)-8-Hydroxy-4-methyl-1-(6-methylpyridin-2-yl)-5,6-dihydro-4H-2,3,6,10b-tetraazabenzene And [e] the preparation of azulene ("A1") was carried out in a scheme similar to the following scheme:
[0202]
[0203] 1.1 Preparation of 5-methoxy-2-nitroaniline
[0204] Dissolve 10 g of 5-chloro-2-nitroaniline in 100 ml of methanol, and add 32.3 g of sodium methoxide. The reaction mixture was refluxed for 18 hours under boiling. After cooling, the mixture was evaporated to dryness, 500 ml of water were added and the crude product was isolated by filtration. After drying, 9.15 g of 5-methoxy-2-nitroaniline are obtained.
[0205] 1.2 Preparation of (R, S)-3-(5-methoxy-2-nitrophenylamino)-2-methyl-propionic acid
[0206] 9.15 g of 5-methoxy-2-nitroaniline were dissolved in 60 ml of THF, 6.5 ml of 2-methacrylonitrile and 1.35 ml of a 40% solution of benzyltrimethylammonium hydroxide in methanol were added. The reaction mixture was heated at the boil for about 20 hours and...
Embodiment A
[0244] Example A: Injection Vials
[0245] A solution of 100 g of the active ingredient of formula I and 5 g of disodium hydrogen phosphate in 3 L of double-distilled water was adjusted to pH 6.5 with 2N hydrochloric acid, sterile filtered, transferred to injection vials, lyophilized under sterile conditions and sterilized. condition sealed. Each injection vial contains 5 mg of active ingredient.
Embodiment B
[0246] Example B: Suppositories
[0247] A mixture of 20 g of the active ingredient of formula I with 100 g of soy lecithin and 1400 g of cocoa butter was melted, poured into moulds and allowed to cool. Each suppository contains 20 mg of active ingredient.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com