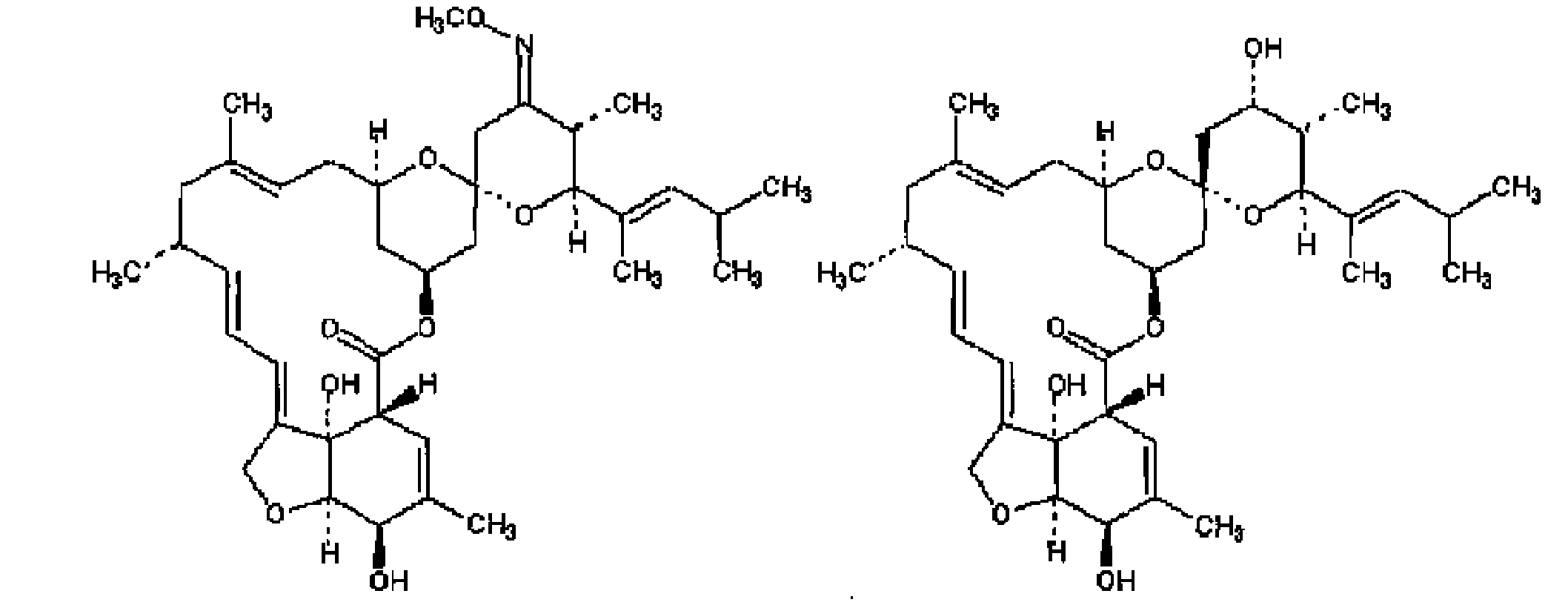
Method for preparing high-purity moxidectin
A high-purity, pre-purification technology, applied in the direction of organic chemistry, drug combination, anti-infective drugs, etc., can solve the problems of complex process, low reusability, large amount of chromatographic solvent, etc., to achieve easy industrialization and improve product purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] Example 1: the method for preparing high-purity moxidectin
[0020] 1, the fermented liquid that Streptomyces fermentation produces is through solid-liquid separation, and the nemoctine prepurification liquid 100g that obtains after leaching, purity 15%, flows through macroporous adsorption resin column, resin model HP-20 (40 order, Mitsubishi Chemical, Japan), the packing volume in the column is 10L, after the flow is completed, use the ethanol aqueous solution gradient elution, from 50%-90% (V / V) gradient elution, control the elution flow rate 1-1.5 times the column per hour volume, collect the nemoctine fraction, add 1 / 10 volume of dichloromethane to extract the eluted fraction, and then concentrate to dryness under reduced pressure. The concentrate contains 85g of nimoctine, with a content of 46%.
[0021] 2. The above-mentioned nimoctine concentrate was subjected to the upper protection reaction (conventional reaction) to obtain 80 g of the upper protection produc...
example 2
[0024] Example 2: the method for preparing high-purity moxidectin
[0025] 1. The fermented liquid produced by Streptomyces fermentation is subjected to solid-liquid separation, and the pre-purified liquid obtained after leaching has a purity of 18%, and 120g of nimoctine 1.2% (W / W) in the solid, flows through macropores for adsorption Resin column, resin model XAD-1600 (40 mesh, produced by Rohm-Haas Company, the U.S.), the filling volume in the column is 12L, and after the flow is finished, use acetone aqueous solution gradient elution instead, from 50%-80% (V / V) Gradient elution, control the elution flow rate of 1-1.5 times the column volume per hour, collect the nemoctine fraction, add 1 / 10 volume of dichloromethane to the elution fraction for extraction, and concentrate to dryness under reduced pressure. The concentrate contains 98g of nimoctine, with a content of 43.6%.
[0026] 2. The above-mentioned nimoctine concentrate was subjected to the upper protection reaction...
example 3
[0029] Example 3: the method for preparing high-purity moxidectin
[0030] 1. The fermented liquid produced by Streptomyces fermentation is subjected to solid-liquid separation, and the pre-purified liquid obtained after leaching has a purity of 13.8%, and contains 0.8% (W / W) 80.6g of nemoctine in the solid, and flows through the large pores Adsorption resin column, resin type HP-20 (40 mesh, Mitsubishi Chemical, Japan), the volume of the column filling is 10L, after the flow is completed, use 85% ethanol solution to elute, and control the elution flow rate to 1-1.5 times the column per hour volume, collect the nemoctine fraction, add 1 / 10 volume of dichloromethane to extract the eluted fraction, and then concentrate to dryness under reduced pressure. The concentrate contains 68.2 g of nimoctine, with a content of 41.5%.
[0031] 2. The above-mentioned nimoctine concentrate was subjected to the upper protection reaction to obtain 63.8g of the upper protection product, which f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com