Lactose-azithromycin hydrate, preparation and use thereof
A technology of azithromycin and lactobionic acid, applied in the field of medicine, achieves the effects of easier cleaning, good dissolution performance, and less contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
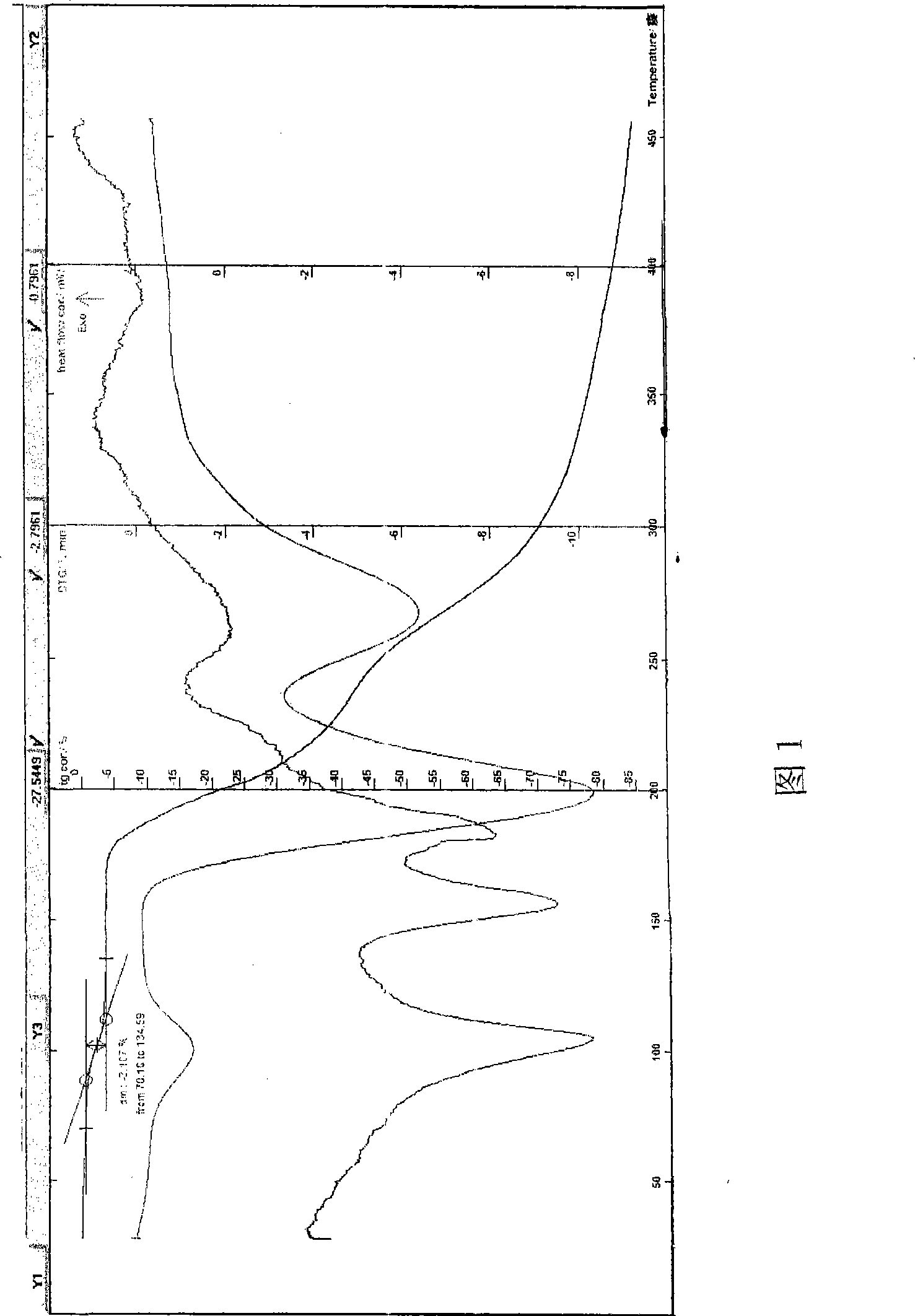
[0055] Example 1 In a three-necked flask, add 100 g of azithromycin dihydrate, add a 7:3 mixed solvent of isopropanol and acetone, stir to dissolve, add 91.2 g of lactobionic acid or its aqueous solution, and keep stirring at about 5 to 10 ° C to make After the reaction is completed, slowly add 1000ml of isopropanol, cool to -20~5°C, wait for the solid to precipitate out, filter, rinse the solid with anhydrous acetone, drain it, and dry it in vacuum at 80°C to obtain an off-white crystalline powder, easily soluble In water, HPLC: the retention time of the main peak of the sample is consistent with that of the main peak of the azithromycin reference substance, melting point: 157-160° C., decomposed (uncorrected), and the moisture measured by the Karl Fischer method is 3.03%, which is consistent with the result that the sample contains 2.5 crystal waters ( Theoretical value 2.98%) is within the error range. TG-DTA: The weight loss of the platform before 130°C is about 3.11%, whi...
Embodiment 2
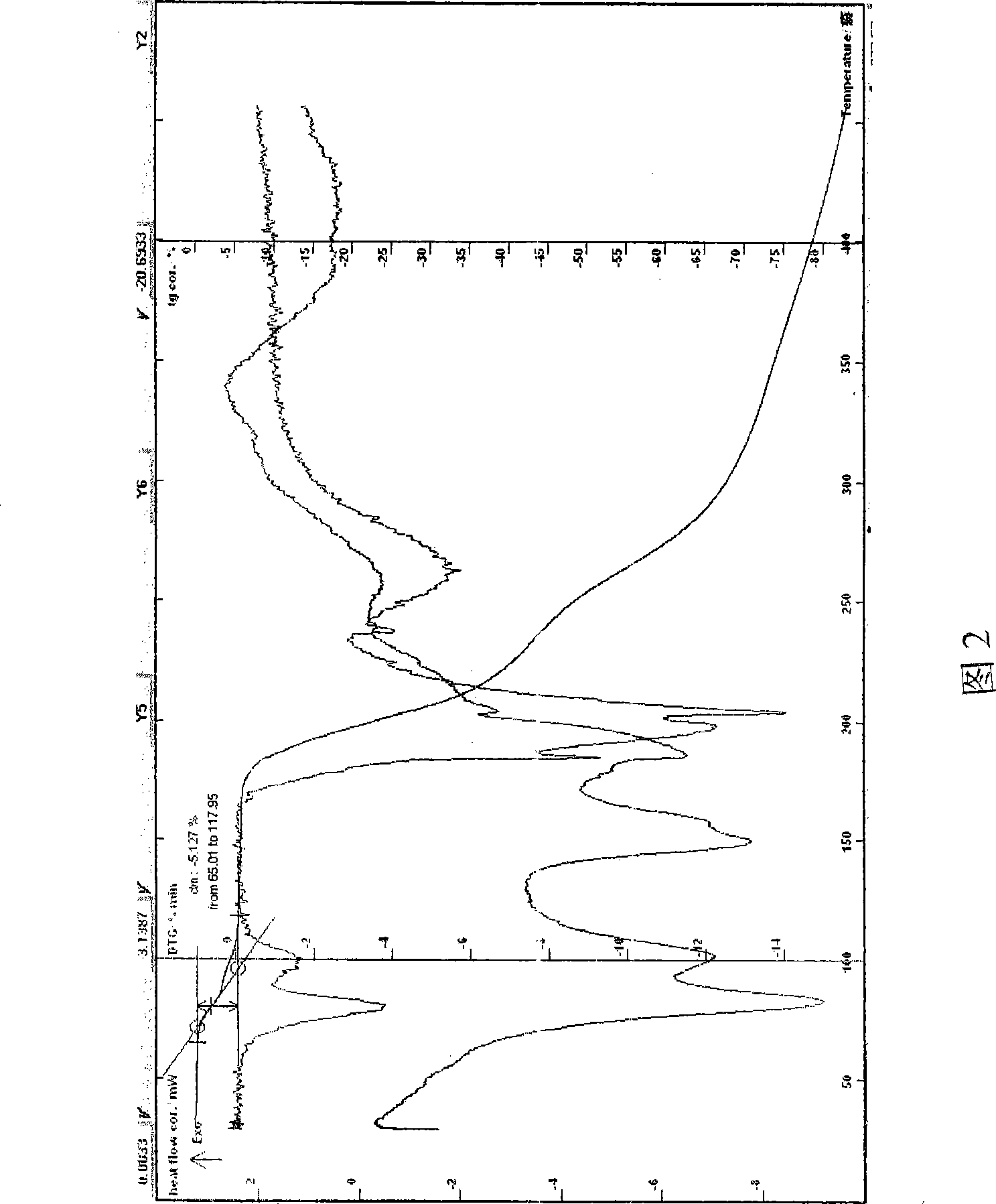
[0056]Example 2 In a three-necked flask, add 95% isopropanol to dissolve 100 g of azithromycin, stir the lactobionic acid at 15-55°C to dissolve, and continue to react for 2 hours. After the reaction is completed, slowly add 2-15 times of formic acid Ethyl ester and isopropanol mixed solvent, cooling, until the solid precipitated, filtered, the solid was rinsed with ethyl formate, drained, and dried at about 50°C to obtain a off-white powder, easily soluble in water, HPLC: the main peak of the sample and azithromycin The retention time of the main peak of the reference substance was the same. Melting point: 151.5-156°C, decomposed, uncorrected; Moisture (Karls method): 5.25%, TG-DTA: The weight loss of the platform before 130°C is about 5.20%, which is consistent with the result that the sample contains 4.5 crystal waters (theoretical value 5.27 %) is within the margin of error. TG-DTA shows that there is an obvious endothermic peak before 130°C (see Figure 2); MS (ESI) m / z: ...
Embodiment 3
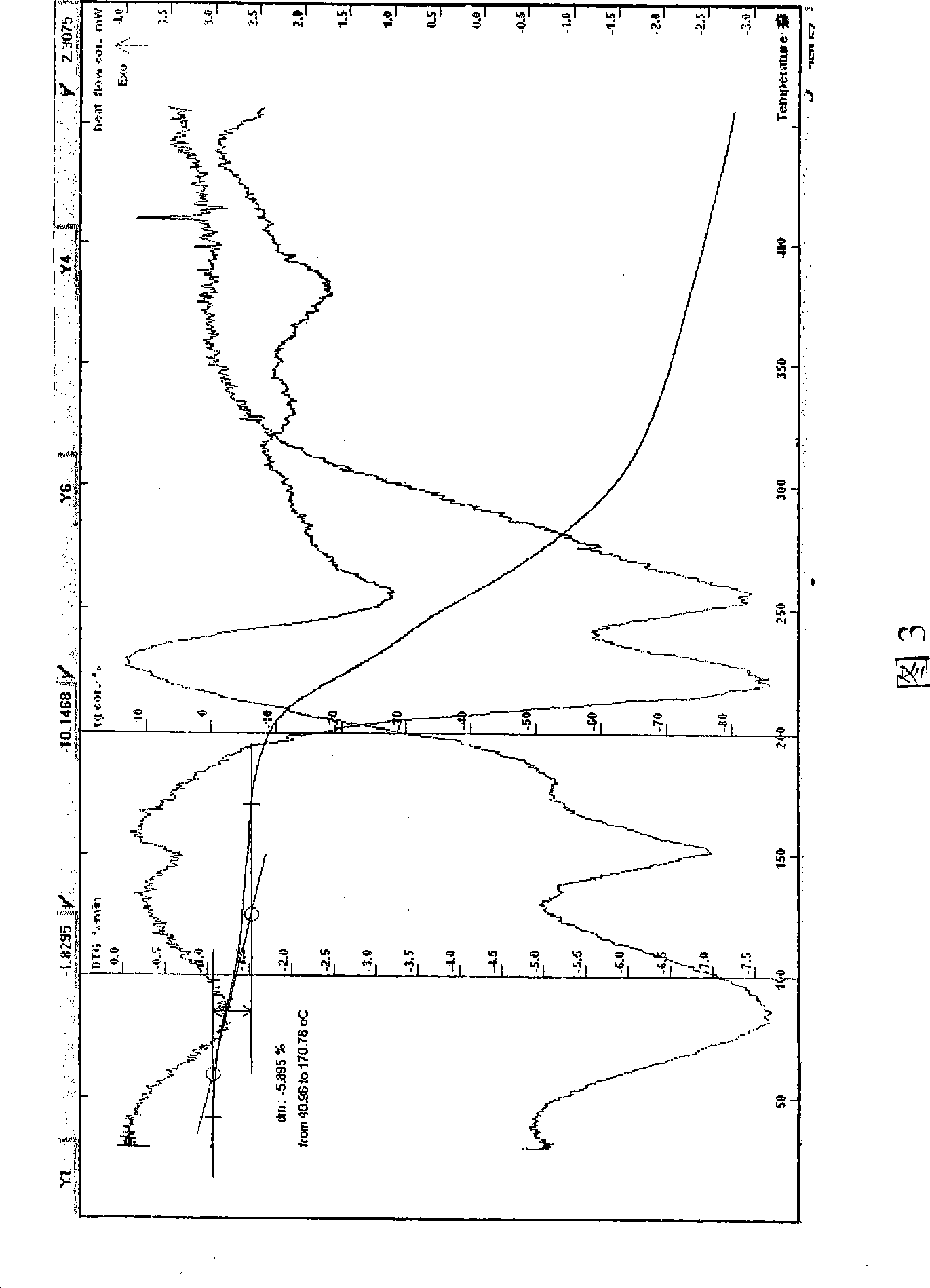
[0057] Example 3 Put lactobionic acid and azithromycin in a molar ratio (2:1) into a reaction flask, add water, stir, control the temperature between 2-20°C, and react for 10-90min. After the reaction is complete, add 0.5% activated carbon and stir For about 30 minutes, filter and decarburize, then use a 0.22 micron microporous membrane to filter aseptically, freeze it to about -50°C, keep it for 6 hours, reduce the temperature of the condenser to -55°C, vacuumize, and let the temperature drop through the shelf. After gradually rising to about -20°C, keep it at about -20°C for about 24 hours, continue heating to raise the temperature of the drug to about 28°C, and keep it for about 6 hours to obtain a off-white powder with a yield of 99%. The sample is easily soluble in water. HPLC: The retention time of the main peak of the sample is consistent with that of the main peak of the azithromycin reference substance, melting point: 153-157°C (uncorrected), decomposed; pH=6.42, the w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com