Pharmaceutical composition for promoting axon regeneration after spinal cord damage and behavior function recovery
A technology of axon regeneration and composition, applied in the field included in the present invention, can solve problems such as clinical trials of rare spinal cord injury cases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Insertion of catheter and infusion of chondroitinase ABC after T8 complete spinal cord transection
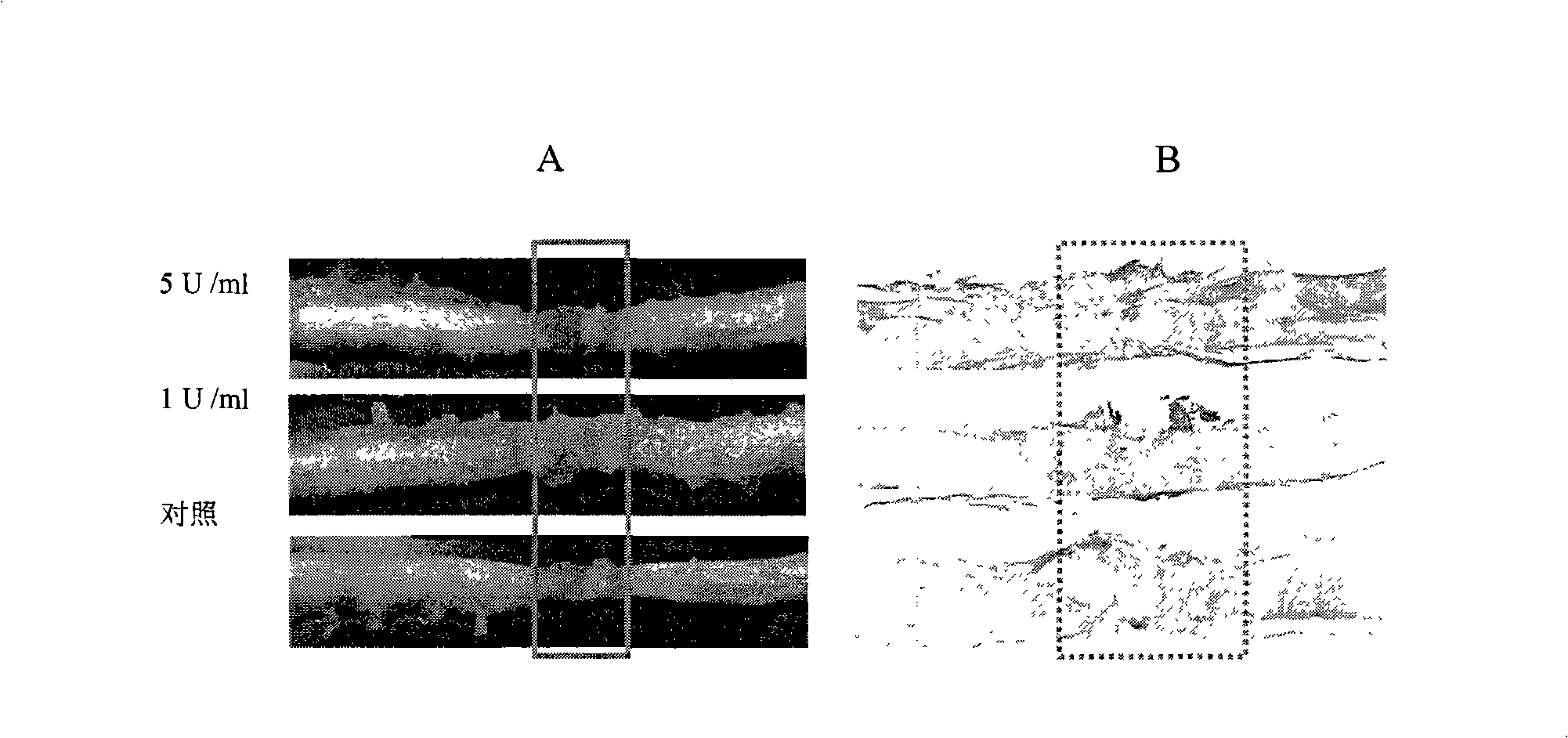
[0032] Female Sprague-Dawley (SD) rats (each weighing 250 to 300 g) were used in this experiment. All surgical procedures were performed under inhalational anesthesia with isoflurane and body temperature maintained at approximately 37°C on a homoeothermic blanket. The skin over the foramina was harvested and a longitudinal midline incision was made to expose the capitate and layer C1. The epidural space is established just above the C1 level, and the catheterization reaches the T8-T9 level. Wounds were closed layer by layer, and surfaced catheters were used to infuse chondroitinase ABC.
[0033] One week after cannulation and confirmation of normal hindlimb movement, the animals underwent a T8 gross laminectomy at the T8 level for exposure of the spinal cord and cannulation. A complete spinal cord transection was performed with the two stumps held up in a m...
Embodiment 2
[0035] Example 2: Evaluation of Behavioral Function
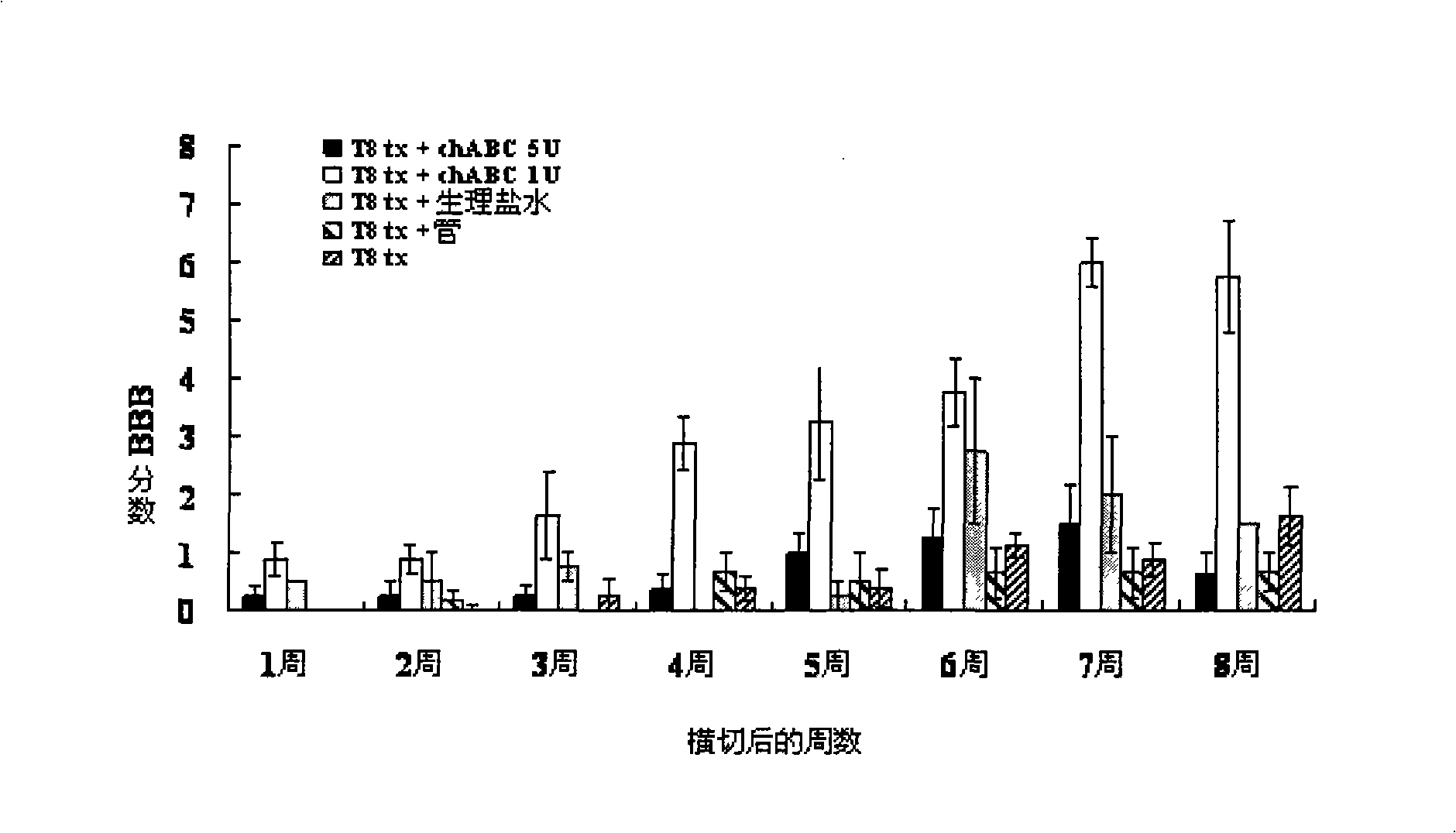
[0036]Weekly after surgery, all animals underwent behavioral function assays for 8 weeks. All behavioral function measurements were videotaped, and the two examiners participating in the behavioral function evaluation were blinded to each group. Rat hindlimb locomotor's behavioral function was evaluated by the Basso, Beattie, Bresnahan (BBB) open (photo) field assay. Each evaluation session lasts 5 minutes. The open (photographed) field locomotor's activity score was determined from 0 to 21 (0 being no movement, 21 being normal movement) by observation and scoring of behavioral functions including trunk, tail and hind limbs.
[0037] refer to figure 1 , after surgery and ChABC treatment, between ChABC 1U / ml group (T8 tx+chABC 1U), ChABC 5U / ml group (T8tx+chABC 5U) and control group (T8 tx+saline, only T8tx+tube and only T8tx) There were statistical differences in the locomotor's functional evaluation of the...
Embodiment 3
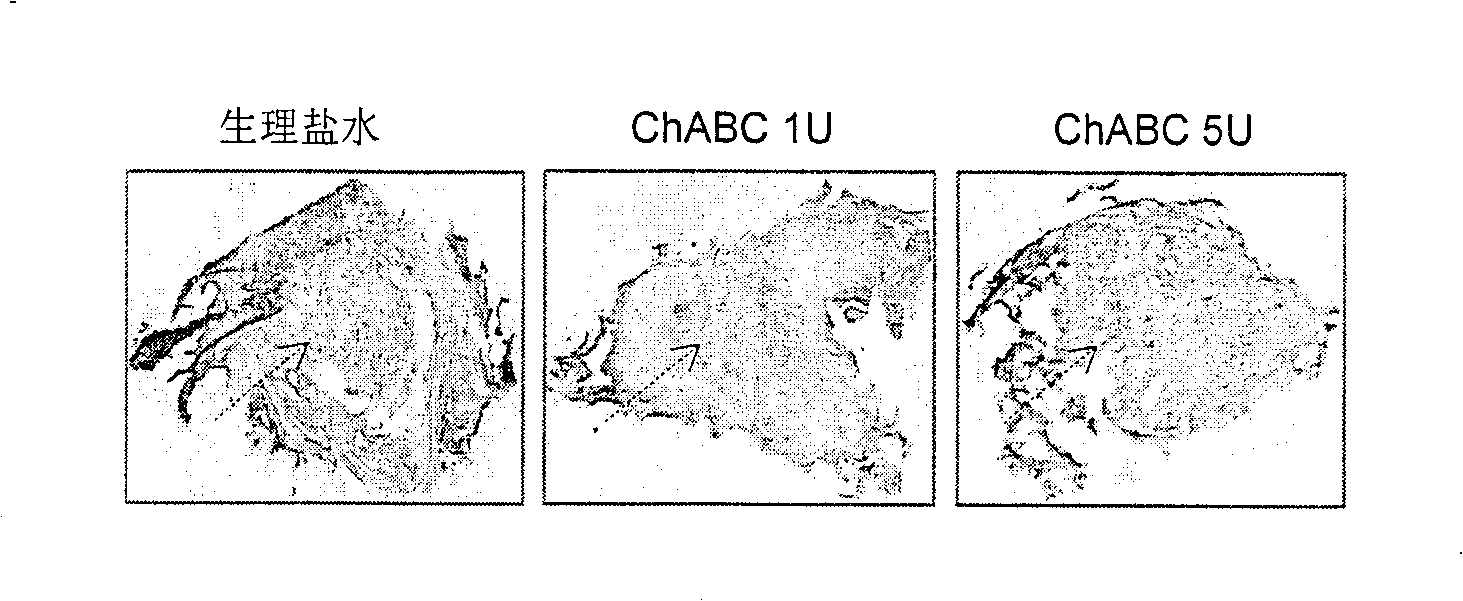
[0038] Example 3: Anterograde labeling of axons passing through the transection site
[0039] Eight weeks later, female SD rats were anesthetized under isoflurane inhalation. The spinal cord was exposed at T10 level, and 4% WGA-HRP was injected into the motor cortex of the brain via a micro-syringe. Three sites were injected by a slow pump system (disperse injection on 0.24 μl x 3 on each side). Two days after microinjection, the animals were sacrificed under anesthesia by carotid infusion of 4% paraformaldehyde. Spinal cords and brainstems were removed, post-fixed and cryopreserved in 30% sucrose overnight for serial sectioning. The spinal cord was sectioned longitudinally and the brainstem was sectioned coronally at a thickness of 30 μm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com