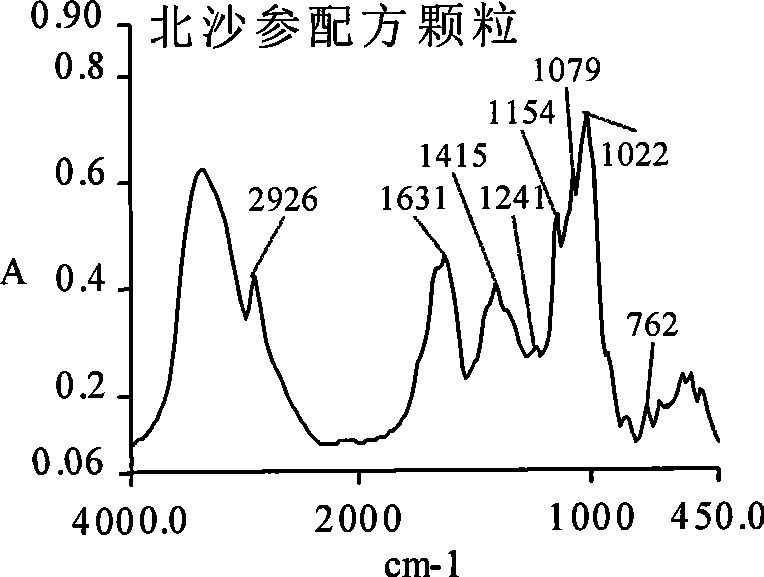
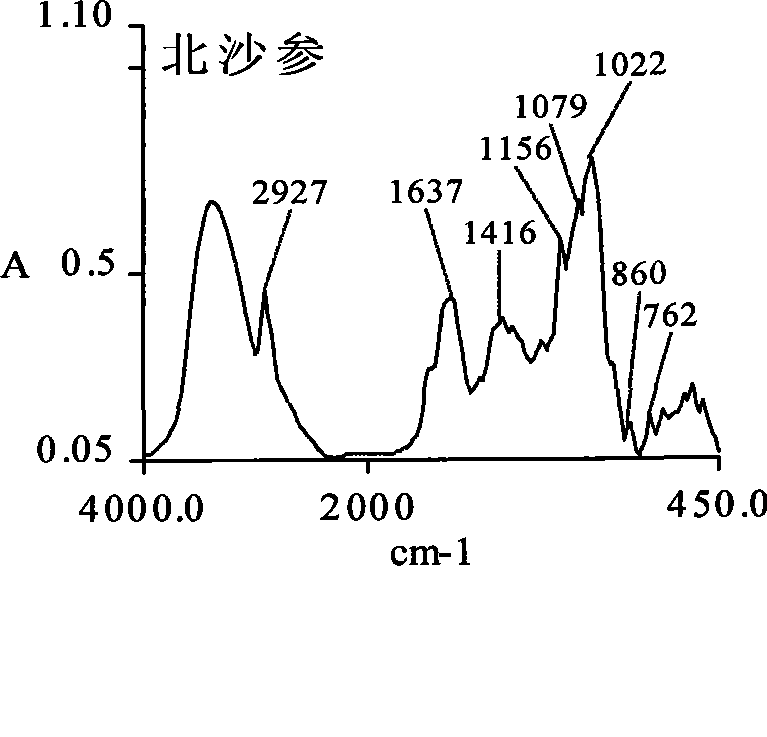
Glehnia root dispensing granule as well as preparation method and quality control method
A technology for formula granules and northern sea ginseng, which can be applied to pharmaceutical formulas, medical preparations containing active ingredients, measuring devices, etc., and can solve problems such as difficulty in spectral analysis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: North Radix Ginseng Formula Granules
[0037] Take 100kg of Adenophora decoction pieces, add 800kg of water, extract for 2 hours, that is, the first decoction; after the first decoction, add 800kg of water to the dregs, extract for 1.5 hours, that is, the second decoction; combine the two decoctions, concentrate under reduced pressure to a relative density of 1.05 —1.10; the concentrated solution is preheated at 45°C, the inlet air temperature is 180°C, the material pump speed is 550 rpm, the outlet air temperature is 80°C, and the air supply temperature is 45°C, and spray-dried to obtain the spray-dried powder of Adenophora japonicus; Add the auxiliary material dextrin of its own weight portion 0-5% in the spray-dried powder of Adenophora japonicus; The pressure of the spray-dried Adenophora japonicus powder is 5MPa on the main roller, the pressure on the side roller is 0.5MPa, and the spindle speed is 400 rpm Dry granulation under the condition that the fee...
Embodiment 2
[0038] Example 2: North Radix Ginseng Formula Granules
[0039] Take 100kg of decoction slices of Adenophora ginseng, add 1000kg of water, extract for 2 hours, that is, the first decoction; after the first decoction, add 600kg of water to the dregs, extract for 1 hour, that is, the second decoction; combine the two decoctions, concentrate under reduced pressure to a relative density of 1.00 —1.05; the concentrated solution is preheated at 50°C, the inlet air temperature is 170°C, the material pump speed is 600 rpm, the outlet air temperature is 75°C, and the air delivery temperature is 50°C, and spray-dried to obtain the spray-dried powder of Adenophora japonicus; Add 5-15% of its own auxiliary material dextrin in the spray-dried powder of Adenophora japonicus; the pressure of the main pressure wheel is 6MPa, the pressure of the side pressure wheel is 0.4MPa, the spindle speed is 450 rpm, and the feeding voltage is 110V dry granulation under certain conditions to obtain 20-60 ...
Embodiment 3
[0041] Embodiment 3: North Radix Ginseng Formula Granules
[0042] Take 100kg of decoction slices of Adenophora ginseng, add 1400kg of water, extract for 2.5 hours, that is, the first decoction; after the first decoction, add 1100kg of water to the dregs, extract for 2.5 hours, that is, the second decoction; combine the two decoctions, concentrate under reduced pressure to a relative density of 1.10 —1.15; the concentrated solution is preheated at 45°C, the inlet air temperature is 170°C, the material pump speed is 600 rpm, the outlet air temperature is 75°C, and the air delivery temperature is 50°C, and the spray-dried powder is obtained; Add 15-25% of its own weight of lactose to the spray-dried powder of Adenophora japonicus; the pressure of the main pressure wheel is 4MPa, the pressure of the side pressure wheel is 0.4MPa, the spindle speed is 500 rpm, and the feeding voltage is 130V. Dry granulation method to obtain 20-60 mesh Adenophora granules formula, each gram of Ade...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com