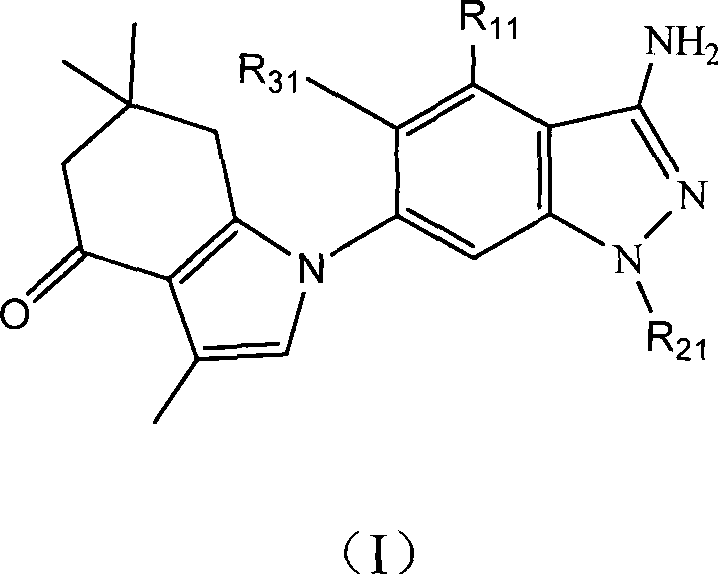
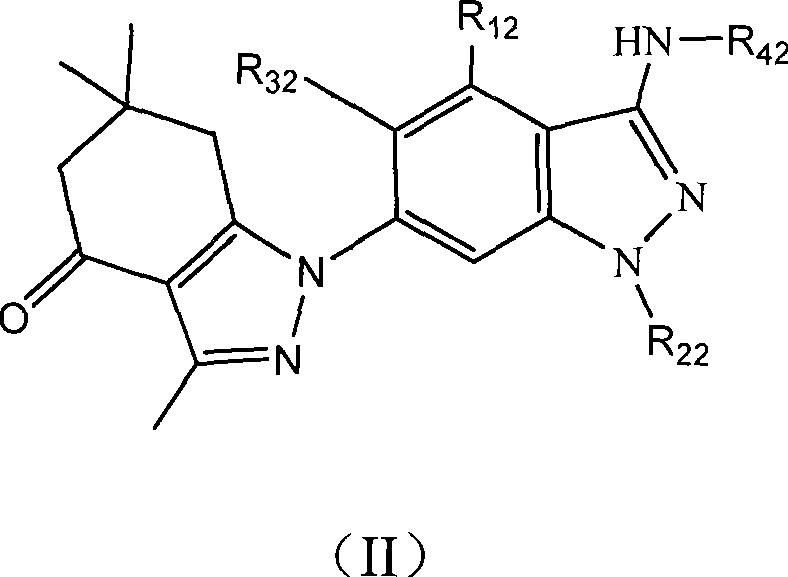
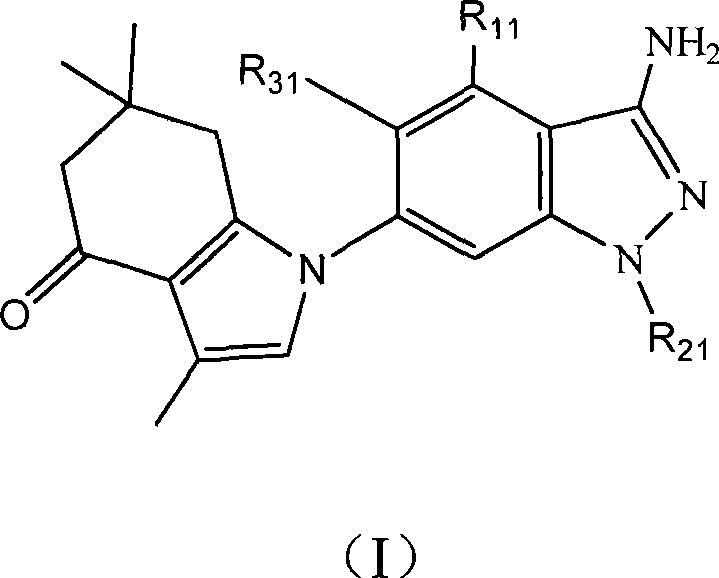
Tetrahydro indazolone or tetrahydro indolone substituted indazole derivative and salt thereof
A technology of tetrahydroindolinone and tetrahydroindazole, which is applied in the field of tetrahydroindazolone or tetrahydroindolinone-substituted indazole derivatives and salts thereof, and can solve the problems of patient toxicity, side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1.1-(6-(3-amino-1-methyl)indazole)-3,6,6-trimethyl-1,5,6,7-tetrahydroindazol-4-one
[0075] (Compound I-01)
[0076] Add 3,6,6-trimethyl-1,5,6,7-tetrahydroindazol-4-one (0.20 g, 1.1 mmol), dry DMF (15 ml), sodium hydride ( 33 mg, 1.3 mmol), A3 (0.3 g, 1.1 mmol) was added with stirring, and the reaction was heated to 150°C and stirred for 2 hours. After cooling, it was extracted with ethyl acetate, washed with saturated NaCl aqueous solution, and dried over anhydrous magnesium sulfate. Filtration, removal of solvent, separation and purification of the product by silica gel column chromatography, eluting with ethyl acetate / cyclohexane (60:40) to obtain 0.34 g of intermediate, which was dissolved in trifluoroacetic acid (15 ml) at room temperature After stirring for 2 hours, the solvent was removed under reduced pressure, extracted with ethyl acetate, washed with saturated NaCl aqueous solution, and dried over anhydrous magnesium sulfate. Filtration, removal o...
Embodiment 2
[0077] Example 2.1-(6-(3-amino-1-propenyl)indazole)-3,6,6-trimethyl-1,5,6,7-tetrahydroindazol-4-one
[0078] (Compound I-02)
[0079] Taking intermediate A4 as starting material, synthesize 1-(6-(3-amino-1-propenyl) indazole)-3,6,6-trimethyl-1,5 with the method in Example 1, 6,7-tetrahydroindazol-4-one, yield 54% (two steps); mp (°C) 173-175; 1 HNMR (500MHz, DMSO-D 6 )δ (ppm): 7.71-7.73 (d, 1H), 7.39-7.41 (b, 1H), 7.11-7.13 (d, 1H), 5.75 (m, 1H), 4.95-4.98 (m, 2H), 3.97 (m, 2H), 3.72(s, 2H), 2.64(s, 3H), 2.42(s, 2H), 2.37(s, 2H), 1.07-1.10(s, 6H); MS 350.2(M + +1).
Embodiment 3
[0080] Example 3.1-(6-(3-amino-1-(3-(1-methylcyclohexyl-4-amino)-4-carbamoylphenyl))indazole)-3,6,6-tri Methyl-1,5,6,7-tetrahydroindazol-4-one
[0081] (Compound I-03)
[0082]According to the method of Example 1, the corresponding 1-(6-(1-tert-butoxy Formyl-3-tert-butoxycarboxamido)indazole)-3,6,6-trimethyl-1,5,6,7-tetrahydroindazol-4-one, yield 78%; mp( ° C) 157-159; 1 HNMR (500MHz, DMSO-D 6 )δ (ppm): 7.92-7.94 (b, 1H), 7.69-7.71 (d, 1H), 7.51-7.53 (d, 1H), 6.78-6.80 (m, 1H), 2.66 (s, 3H), 2.44 (s, 2H), 2.35 (s, 2H), 1.08-1.11 (s, 6H); MS 510.3 (M + +1).
[0083] The above product (0.25 g, 0.4 mmol) was dissolved in trifluoroacetic acid (15 mL) to remove tert-butoxyformyl to give the corresponding 1-(6-(1H-3-amino)indazole)-3,6,6 - Trimethyl-1,5,6,7-tetrahydroindazol-4-one 0.1 g, yield 80%. 1-(6-(1H-3-amino)indazole)-3,6,6-trimethyl-1,5,6,7-tetrahydroindazol-4-one (0.1 g, 0.3 mmol ) was dissolved in dry DMF (15 ml), sodium hydride (10 mg, 0.4 mmol) was added, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com