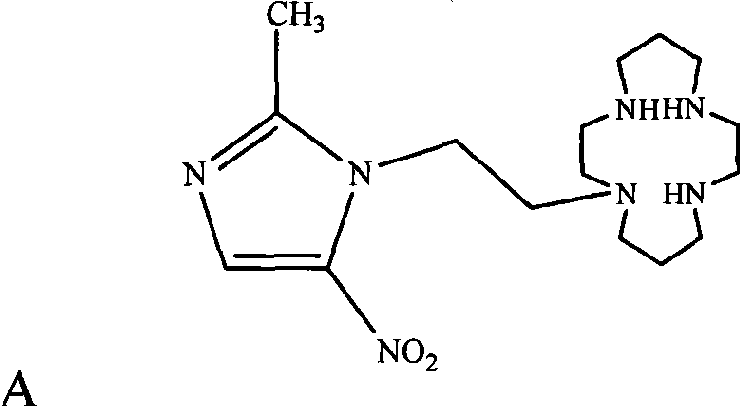
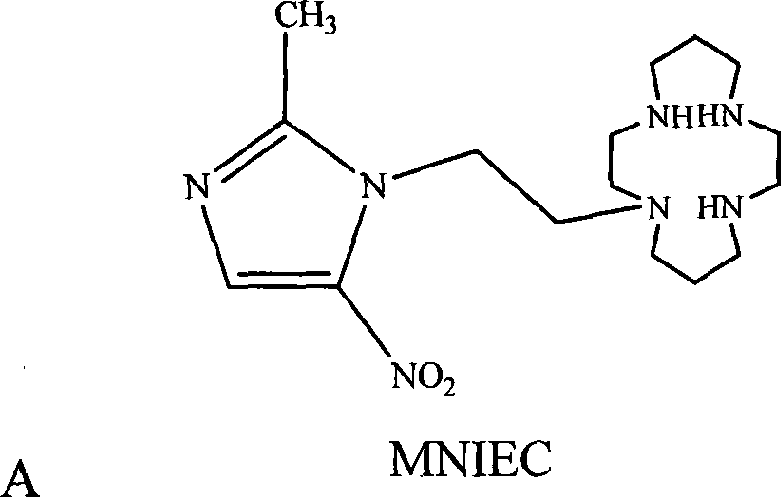
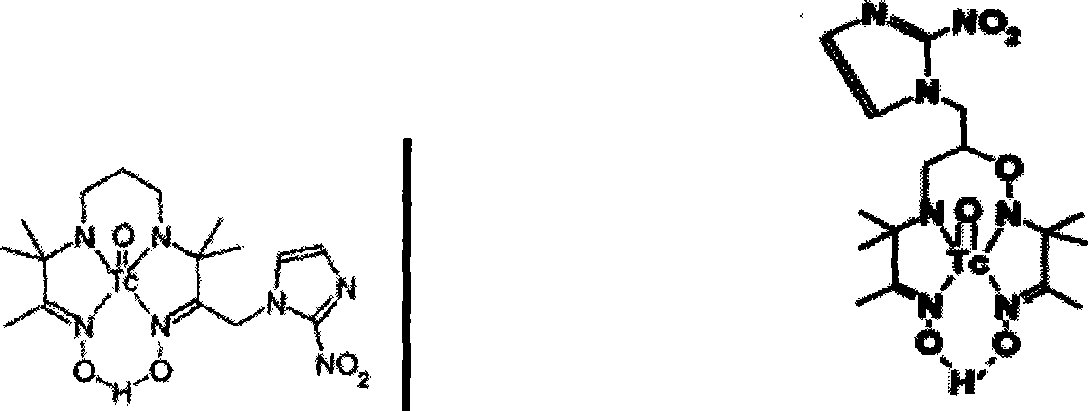2-methyl-5-nitro glyoxaline compound, preparation and use thereof
A technology of nitroimidazoles and nitroimidazoles, applied in the application field of nitroimidazole complexes and their precursors in tumor imaging agents, to achieve high tumor uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Compound MNIEC provided by the invention is synthesized according to the synthetic route shown below:
[0034]
[0035] The reagents used in each step are:
[0036] (a) EtOTFA, Et 3 N and MeOH;
[0037] (b) 2-bromoethanol, K 2 CO 3 and CH 3 CN;
[0038] (c)CH 3 SO 2 Cl, CH 2 Cl 2 and Et 3 N;
[0039] (d) Compound 1, K 2 CO 3 and CH 3 CN;
[0040] (e) NaOH and MeOH
[0041] Specific steps are as follows:
[0042] Synthesis of Tri-TFA cyclam (compound 1):
[0043] In a dry 50mL three-necked flask, cyclam (1.0g, 5mmol) and triethylamine (0.69mL, 5mmol) were dissolved in 20mL of anhydrous methanol, nitrogen protection was introduced and the reaction solution was kept at 0-5 with an ice-water bath. ℃, then added ethyl trifluoroacetate (6.0mL, 50.0mmol) dropwise, continued the dropwise addition for ten minutes, stirred and reacted for 7 hours, suspended and evaporated to remove the solvent, the residue was passed through a small silica gel column, and eth...
Embodiment 2
[0052] preparation 99m TcO-MNIEC complex
[0053] 1) 99m Preparation of TcO-GH
[0054] Weigh 25 mg of GH ligand, dissolve it completely with 0.5 mL of normal saline, add 0.5 mL of SnCl 2 2H 2 O (1mg / mL, 0.1M HCI), adjust pH=7-7.5, add 0.5mL of freshly rinsed 99m TCO 4 - The solution (approximately 10 mCi) was heated in a water bath at 40° C. for 10 minutes, then cooled to room temperature, and prepared as a precursor for the ligand exchange method.
[0055] 2) 99m Preparation of TcO-MNIEC
[0056] Dissolve 20 mg of the compound MNIEC ligand in 20 mL of methanol, take out 0.15 mL and put it into a penicillin vial, adjust the pH value to 13 with 1.0 mL of pH=13, that is, 0.1 M NaOH buffer solution, and add 0.15 mL of 99m Tc-GH solution, the mixture was heated in a boiling water bath for 45 minutes, and filtered through a filter membrane to obtain an electrically neutral and water-soluble target complex, and the labeling rate was greater than 95%.
Embodiment 3
[0058] preparation 99m TcN-MNIEC complex
[0059] (1) Intermediate [ 99m TcN] 2+ preparation of
[0060] Can be prepared in two ways 99m TcN intermediates, namely
[0061] Take 1~2mL 99m TCO 4 - Add the fresh eluent to the DTCZ kit, shake well, and heat in a boiling water bath for 15 minutes to obtain the intermediate [ 99m TcN] 2+ , the marking rate is greater than 98%;
[0062] or
[0063] Take 1~2mL 99m TCO 4 - Add the fresh eluent into the SDH kit, shake well, and let it stand at room temperature for 15 minutes to obtain the intermediate [ 99m TcN] 2+ , The labeling rate is above 98%.
[0064] (2) 99m Preparation of TcN-MNIEC
[0065] Take 0.1mL of the ligand solution MNIEC prepared above and put it into a penicillin vial, adjust the pH value of the MNIEC ligand solution to 13 with 1.0mL of 0.1M NaOH buffer solution, and then add 0.1mL[ 99m TcN] 2+ For the intermediate, heat in a boiling water bath for 40 minutes to obtain an electrically neutral and wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com