Composition containing tripterine, preparation method and use
A technology of tripterine and composition, which is applied in the field of medicine and can solve problems such as poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
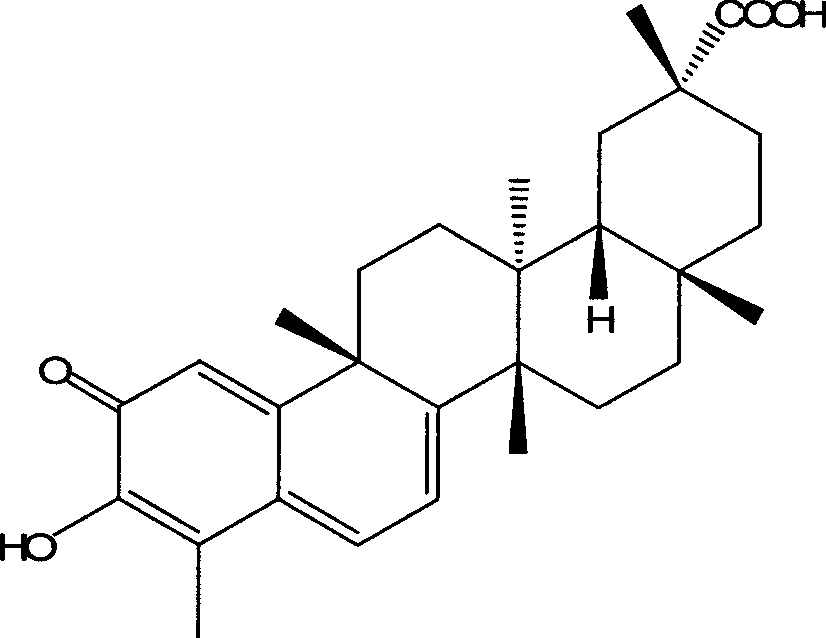
[0018] Dissolve 2.25g of tripterine in 200ml of absolute ethanol, dissolve 1.74g of arginine in 10ml of water, add the aqueous solution to the ethanol solution, stir at room temperature for 2 hours until clear, and rotate the film under vacuum at 50°C The solvent was recovered by the evaporator to dryness, and the solid powder was 3.67 g in total.
Embodiment 2
[0020] Dissolve 2.25g of tripterine in 200ml of absolute ethanol, dissolve 2.61g of arginine in 20ml of water, add the aqueous solution to the ethanol solution, stir at room temperature for 1 hour until clear, and rotate the film in vacuum at 40°C The solvent was recovered by the evaporator to dryness, and the total solid powder was 4.57g.
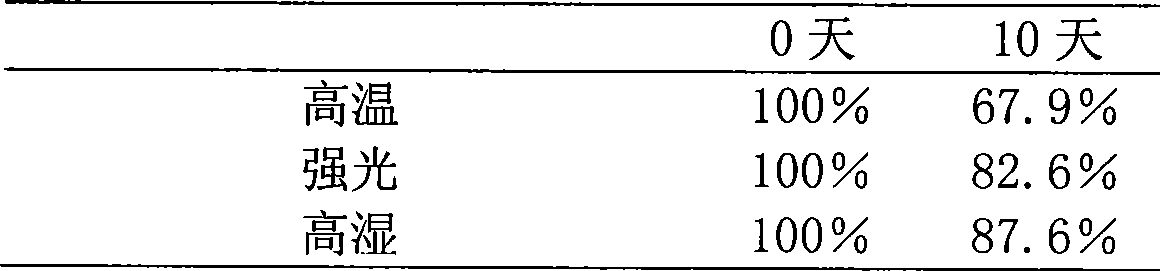
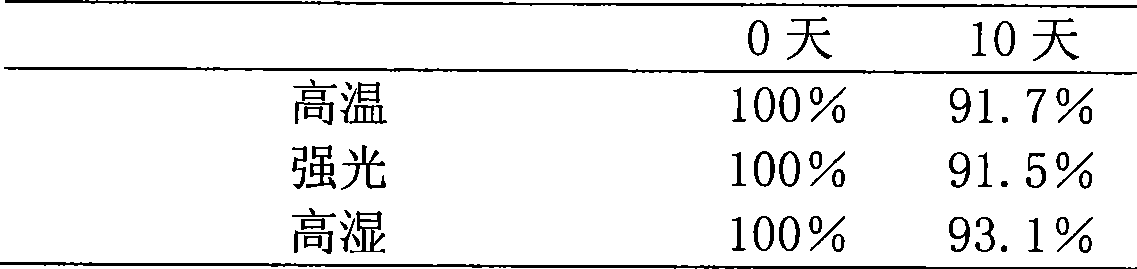
[0021] Take 4.32 g of the solid powder obtained in this example, dissolve it in water for injection, add 2 g of mannitol as an excipient, add water for injection to 100 ml, filter and sterilize with a microporous membrane, and dispense 2 ml into 10 ml of cillin The bottle is freeze-dried to obtain the freeze-dried powder injection, and the specification is 40 mg / bottle in terms of tripterine.
Embodiment 3
[0023] Dissolve 2.25g of tripterine in 400ml of methanol, dissolve 0.73g of lysine in 10ml of water, add the aqueous solution to the methanol solution, stir at 30°C for 2 hours until clear, freeze-dry at -40°C for 24 hours, that is Obtained, a total of 2.84g of solid powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com