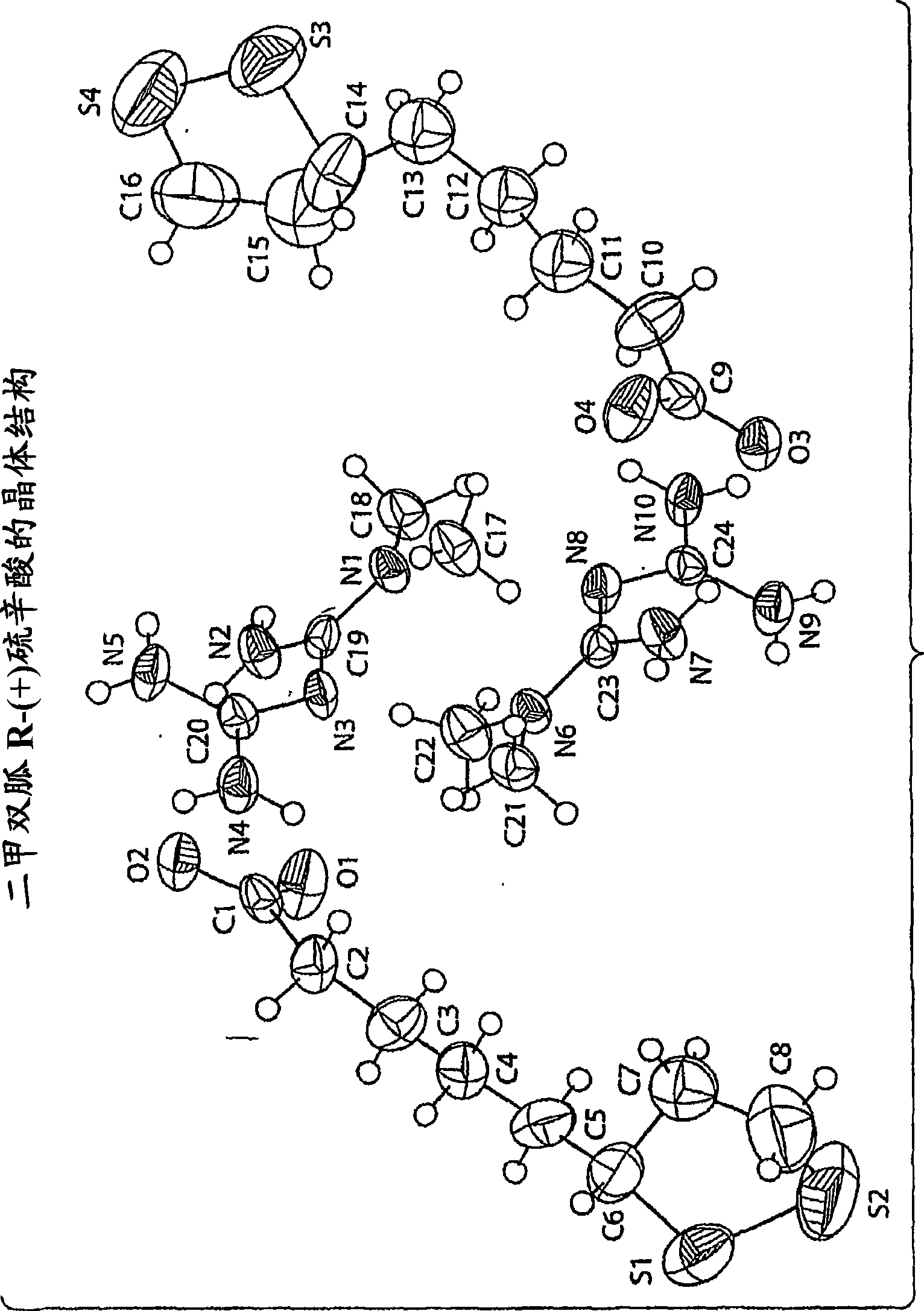
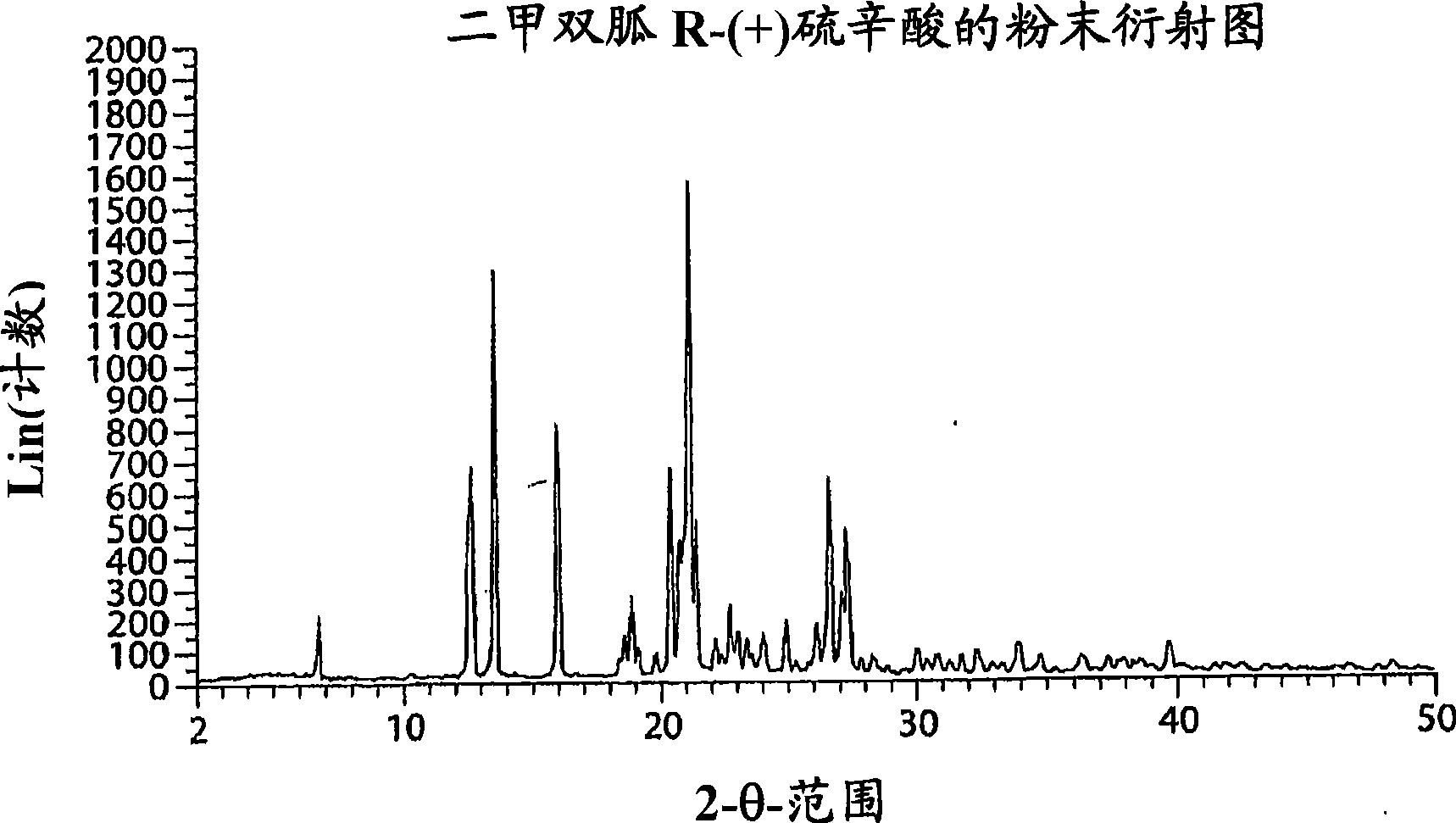
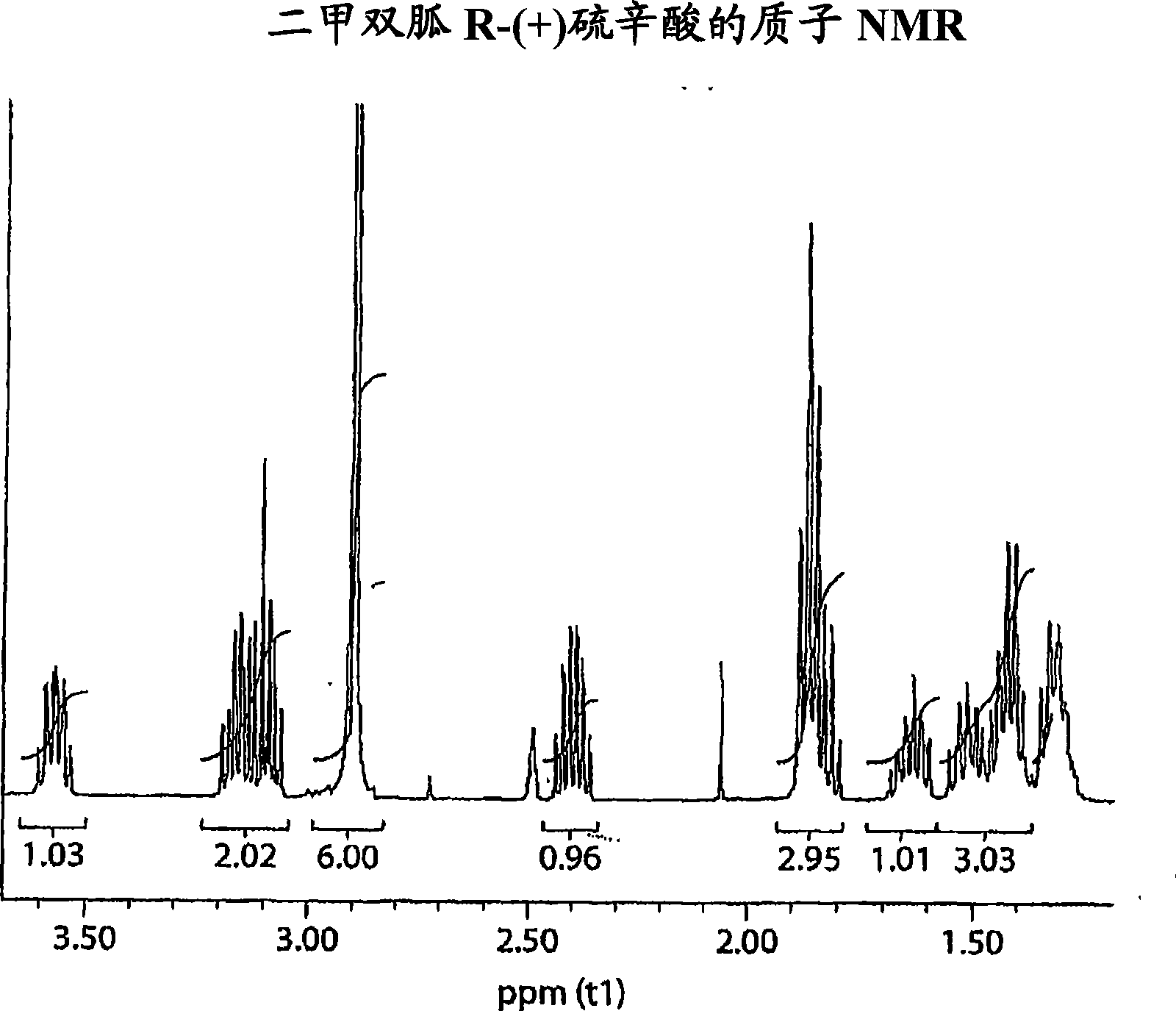
Metformin r-(+) lipoate as an antidiabetic agent for control of diabetic hyperglycemia and diabetic complications
一种二甲双胍、硫辛酸盐的技术,应用在作为抗糖尿病药用于控制糖尿病高血糖症和糖尿病并发症的二甲双胍R-(+)硫辛酸盐领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Preparation of metformin R-(+)-lipoate
[0066] Step 1.1 Preparation of metformin free base:
[0067] Amberlyst A26(OH) ion exchange resin (60 g) was washed 3 times with deionized water and 2 times with 2% (v / v) deionized water in methanol. The resin was packed into a 2.5 cm diameter glass column (packed column height 20 cm). The column was further rinsed with 300 mL of 2% (v / v) deionized water in methanol. Metformin hydrochloride (6.5 g) was dissolved in 180 mL of 2% (v / v) deionized water in methanol and eluted through an ion exchange column. The column was further washed with 200 mL of 2% (v / v) deionized water in methanol. The combined eluates were concentrated under reduced pressure to give a white powder. The powder was dissolved in acetone, filtered through celite and the eluate containing metformin free base was concentrated under reduced pressure.
[0068] or
[0069] In a beaker, Amberlyst A-26(OH) ion exchange resin (60.097) was mixed with ...
Embodiment 2
[0072] Example 2: Preparation of metformin R-(+)-lipoate in acetone
[0073] Dissolve the metformin (free base) prepared in step 1.1 above in 70 mL of acetone. Dissolve R-(+)-lipoic acid in 10 mL of acetone. The two solutions were combined and stirred for 10 minutes. The resulting precipitate was filtered and the solid residue was vacuum dried to afford metformin R-(+)-lipoic acid salt (yield 95%).
Embodiment 3
[0074] Example 3: Preparation of metformin R-(+)-lipoic acid salt in acetonitrile
[0075] Dissolve the metformin (free base) prepared in step 1.1 above in 50 mL of acetonitrile. R-(+)-lipoic acid was dissolved in 20 mL of acetonitrile. The two solutions were combined and stirred for 10 minutes. The resulting precipitate was filtered and the solid residue was vacuum dried to afford metformin R-(+)-lipoic acid salt (yield 95%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com