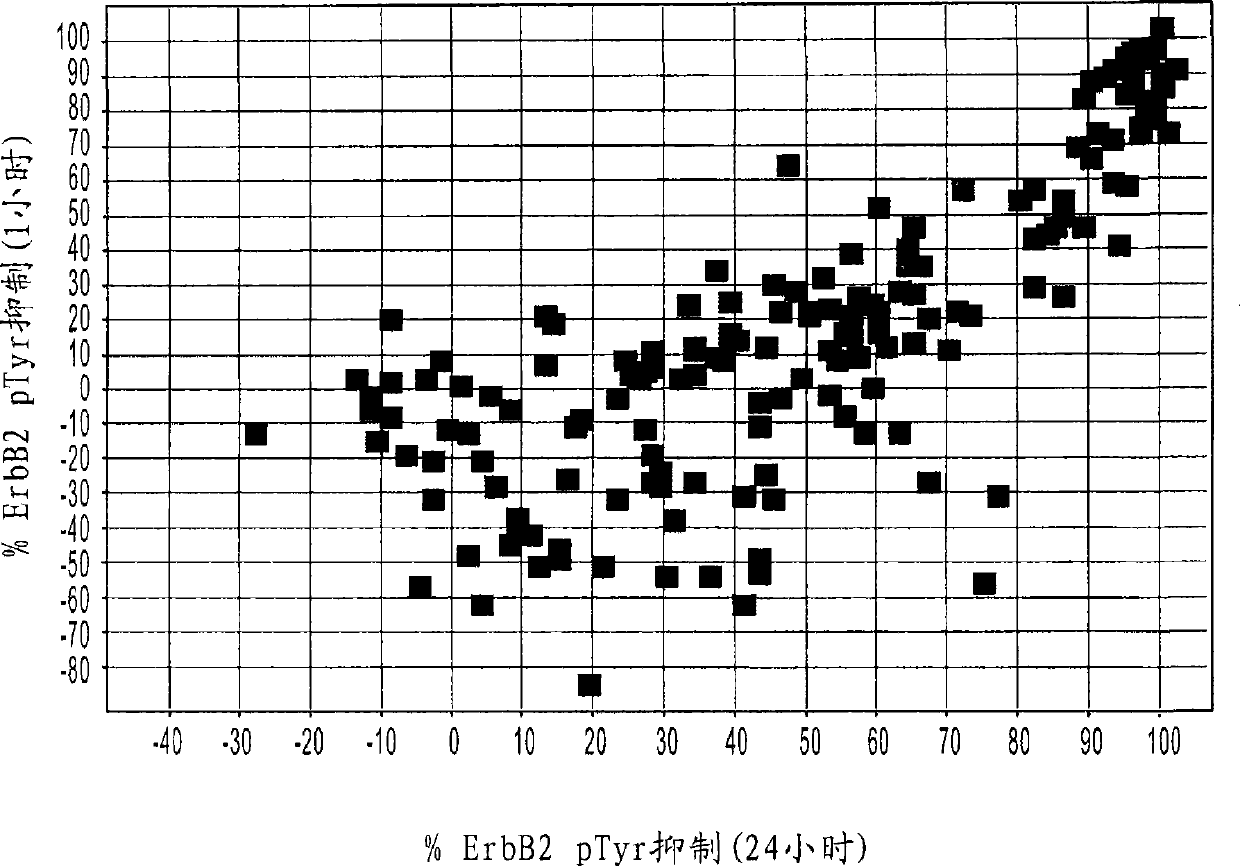
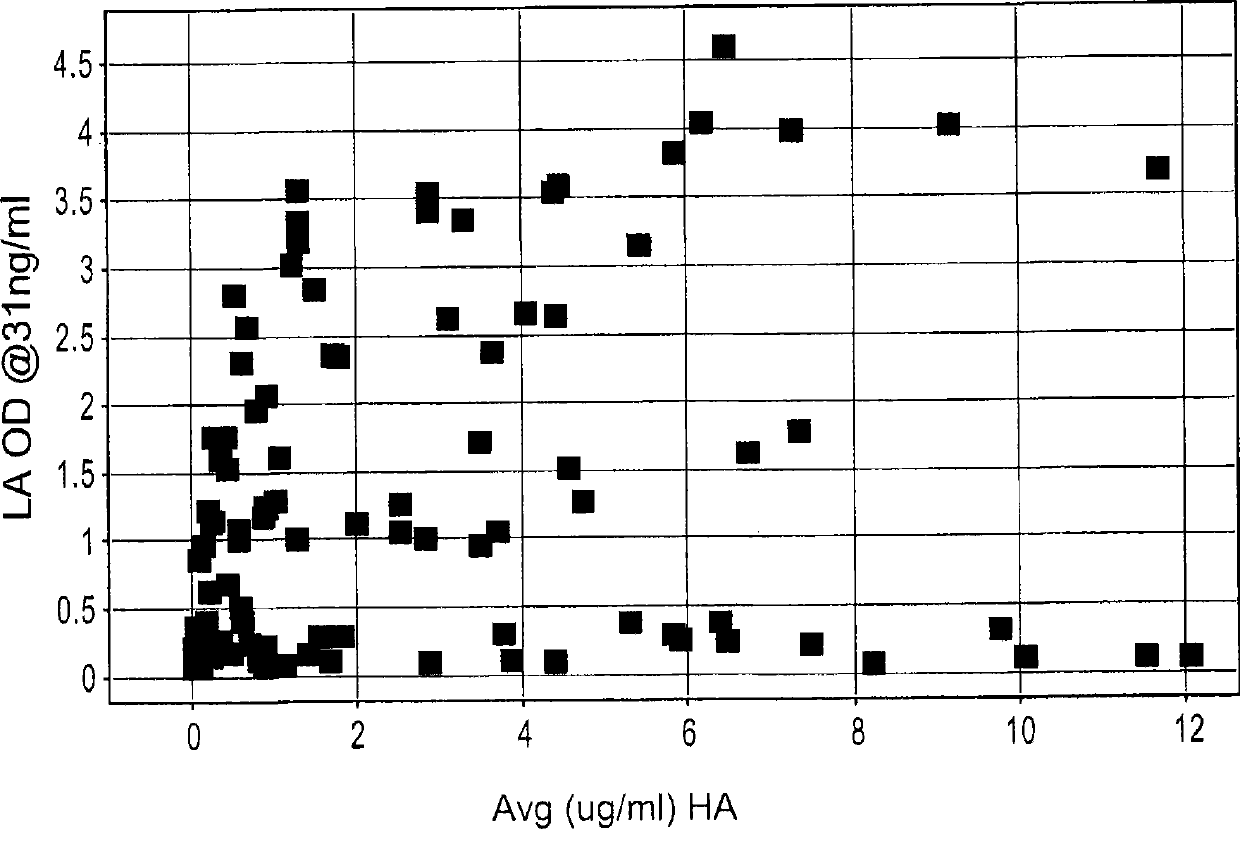
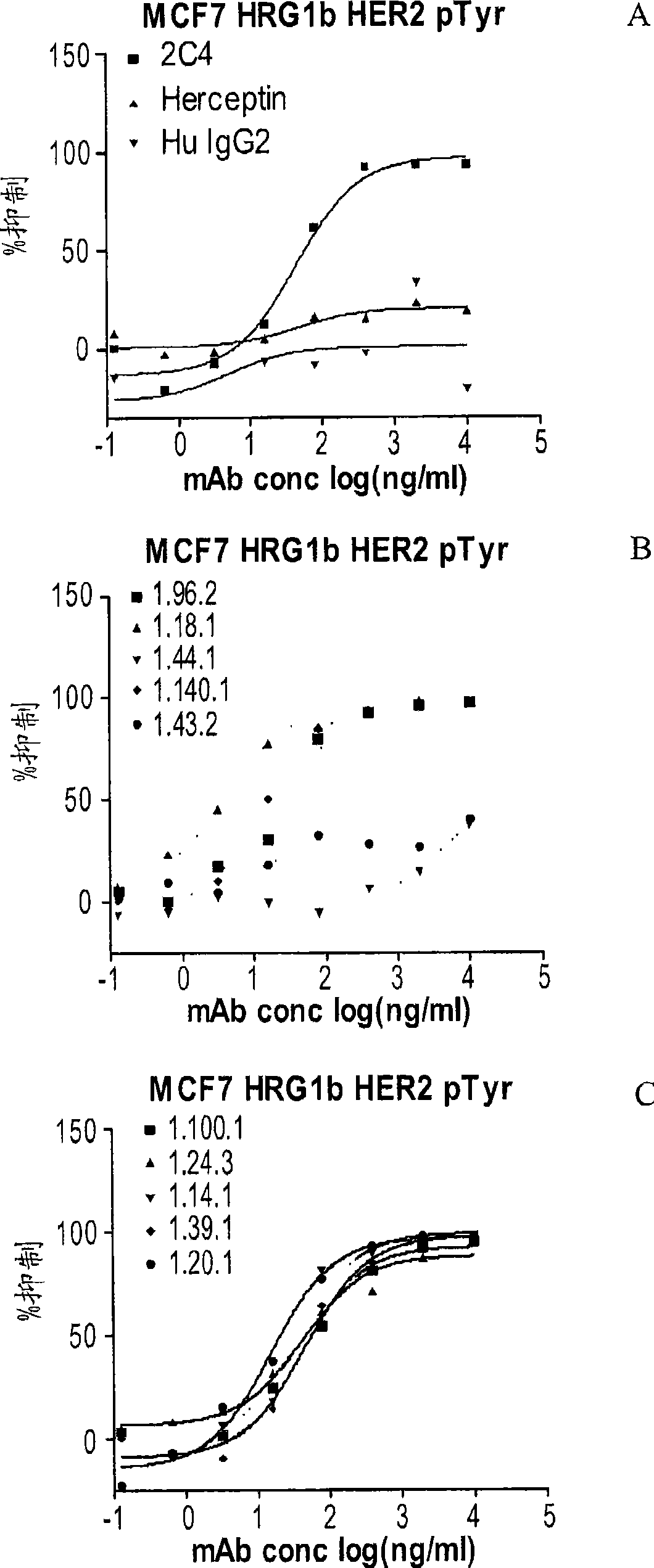
Antibodies to erbb2
A technology of antibody and monoclonal antibody, applied in the field of preparation and use of anti-ErbB2 antibody such as to treat cancer, human antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0394] Immunization and Titration
[0395] Immunization
[0396] A recombinant human ErbB2-ECD / Fcγ1 fusion protein comprising the extracellular domain of human ErbB2 and the Fc region of human IgG1 was obtained from R&D Systems, Inc. (Minneapolis, MN catalog #1129-ER / CF) for use as an immunogen. Monoclonal antibodies against ErbB2 were sequentially immunized by injection via the footpad route Mice (XenoMouse strainXM3B-3, Abgenix, Inc. Fremont, CA) were developed. The first injection was with 10 μg of recombinant human ErbB2-ECD / Fcγ1 per mouse in Titermax Gold (Sigma, catalog #T2684, lot #K1599). Subsequent 10 boosts were performed with 10 μg recombinant human ErbB2-ECD / Fcγ1 per mouse in 15 μl qCpG (ImmunEasy Mouse Adjuvant, Cat #303101; Qiagen), mixed with 5 μl Adju-Phos (Aluminophosphate Gel, Cat #1452- 250, HCI Biosector) mixed. The total volume per injection was 50 μl / mouse, 25 μl / footpad. Mice were immunized twice a week for 5 weeks and fusions were performed on...
Embodiment 2
[0404] Lymphocyte recovery, B cell isolation, fusion and hybridoma generation
[0405]Lymph nodes (LN) were harvested from immunized mice and processed into 3 ml sterile FASC buffer (PBS, 2% FBS). LN cells were filtered through a 40 μm cell strainer, spun down at 400 g for 3 minutes and resuspended in 3 ml fresh FACS buffer. Cells were counted and biotinylated antibodies against CD90 (Pharmingen, Cat #553002), CD4 (Pharmingen, Cat #553728), CD8 (Pharmingen, Cat #553029) and IgM (Pharmingen, Cat #555781 ) were subsequently added. Cells and the above antibodies were gently mixed and incubated on ice for 10 minutes. Cells were spun down again and washed 1x with FACS buffer. SA Dynal beads (M-280) were added to the cells at a 4:1 bead to target cell ratio and incubated for 12 minutes at room temperature with rotation. Place the cells / beads in the 15ml tube in the magnetic field of the Dynal magnet for 2 minutes. The supernatant containing the IgM-fraction was transferred to ...
Embodiment 3
[0409] Antibody screening by FMAT / FACS
[0410] After 14 days of culture, hybridoma supernatants were screened for ErbB2-specific antibodies by FMAT (Fluorometric Microvolume Assay Technology). Briefly, 4275 B300.19 / hErbB2 (positive cells) or B300.19 (ErbB2-negative cells) cells were incubated with 400 ng / ml Cy5 goat anti-human IgG (JIR, cat #109) in 15 μl FACS buffer. -176-098) were mixed and then incubated with 15 μl hybridoma supernatant for 3 hours at room temperature. Positive control antibodies were anti-hErbB2(2C4) with Cy5 goat anti-mouse IgGγ (JIR, catalog #115-176-071 ) combined with SA-Cy5 (JIR catalog #016-170-084, 350 ng / ml) or Goat anti-mouse IgG-Biot (Southern Biotechnology / SB Cat #1030-08, 400 ng / ml) was used for detection. Anti-KLH G1 antibody (Gmix, in-house) was used as isotype control. Plates were read on a FMAT 8100 HTS system from Applied Biosystems. Fluorescent signal and counts were determined after data analysis, and 362 positive hybridomas showi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com