Composition for two-component fluorine coating material
一种组合物、涂料的技术,应用在防腐涂料、聚脲/聚氨酯涂料、涂层等方向,能够解决损伤旧涂膜等问题,达到涂膜物性优异、加工性优异、溶解性优异的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
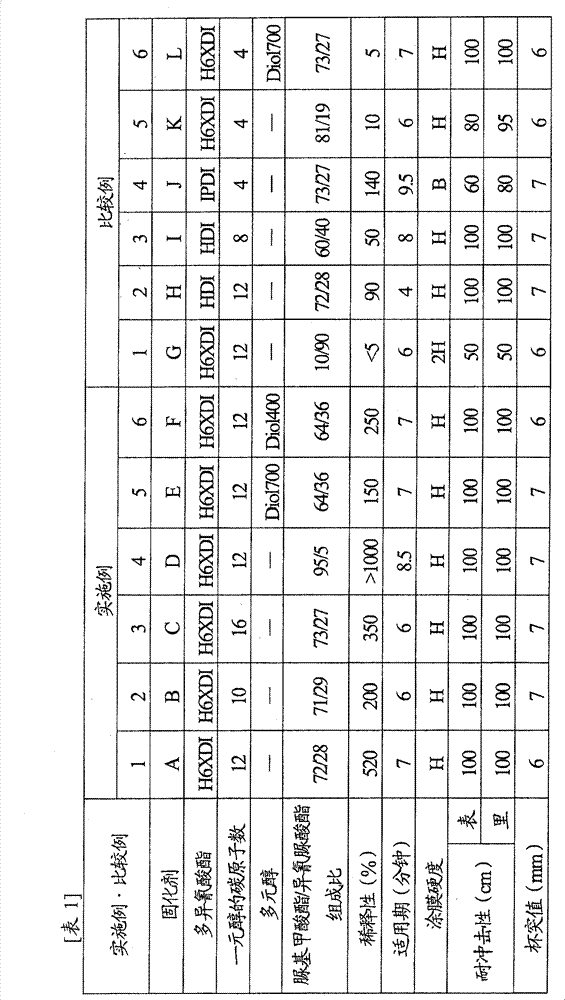
[0095] Next, although this invention is demonstrated based on an Example and a comparative example, this invention is not limited to a following example.
Synthetic example 1
[0096] Synthesis example 1 (synthesis of curing agent A)
[0097] Under nitrogen atmosphere, charge 455.6gH into a 500mL capacity four-necked bottle equipped with stirrer, thermometer, nitrogen inlet tube and Dimro reflux condenser (Dimroth condenser). 6 XDI and 44.4g dodecanol, heated to 90°C and held for 2 hours.
[0098] Afterwards, as a reaction catalyst, add 0.02g of 2-ethylhexanoic acid trimethyl-N-2-hydroxypropyl ammonium (trimethyl-N-2-hydroxypropyl ammonium 2-ethylhexanoate), while adjusting the reaction temperature to 90±5°C , while continuing to react for 2 hours. Then, 0.02 g of o-toluenesulfonamide was added as a catalyst deactivator to deactivate the reaction catalyst and stop the reaction.
[0099] Remove unreacted H from the resulting reaction solution 6 XDI obtained 201.1 g of light yellow and transparent polyisocyanate curing agent A (conversion rate 40%).
[0100] The allophanate / isocyanurate composition ratio of this polyisocyanate curing agent A is 72 / ...
Synthetic example 2
[0102] Synthesis example 2 (synthesis of curing agent B)
[0103] A polyisocyanate curing agent B was obtained in the same manner as in Synthesis Example 1, except that n-decyl alcohol was used instead of dodecyl alcohol as the monohydric alcohol.
[0104] The allophanate / isocyanurate composition ratio of this polyisocyanate curing agent B is 71 / 29, the isocyanate content is 17.0%, the viscosity (by the viscosity measured by BL type viscometer) is 26000mPa·s, unreacted h 6The content of XDI was 0.5% by mass, and it was confirmed by NMR measurement that there was substantially no urethane bond.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com