Method for detecting electrochemical luminescence
A technology of luminescence detection and electrochemistry, applied in chemiluminescence/bioluminescence, organic chemistry, electrical excitation analysis, etc., can solve the problem that the intensity of electrochemiluminescence is not the strongest, and achieve the effect of high electrochemiluminescence efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
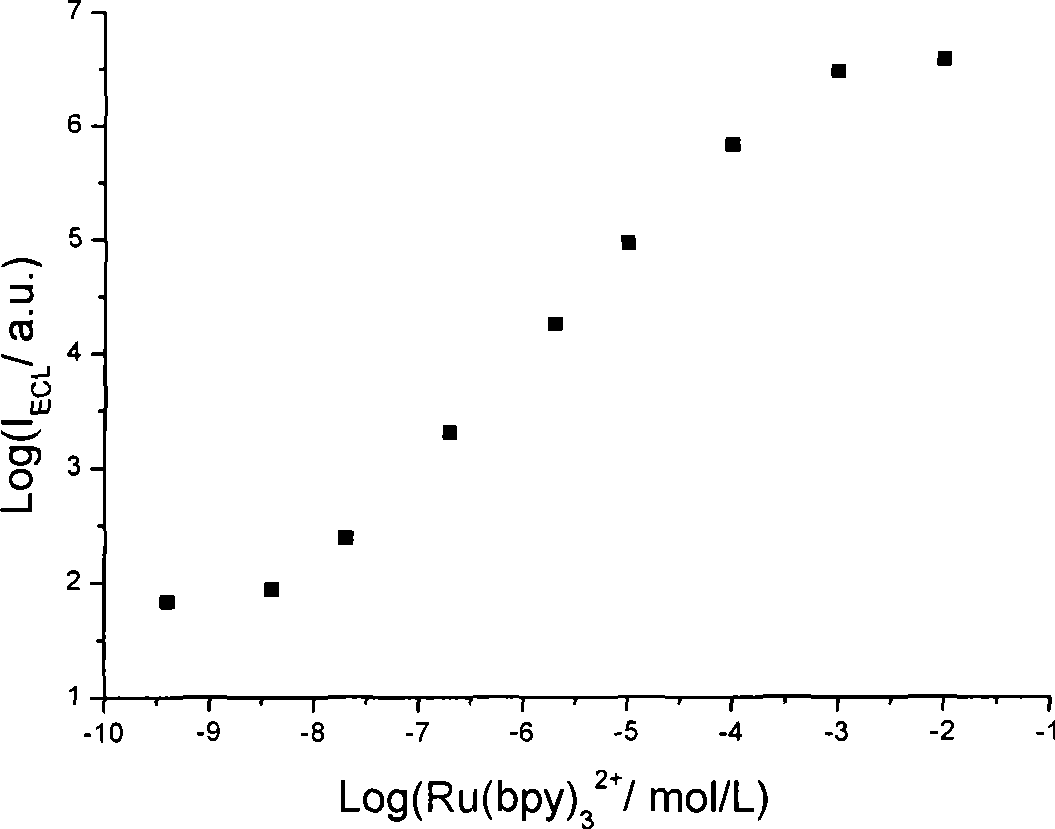
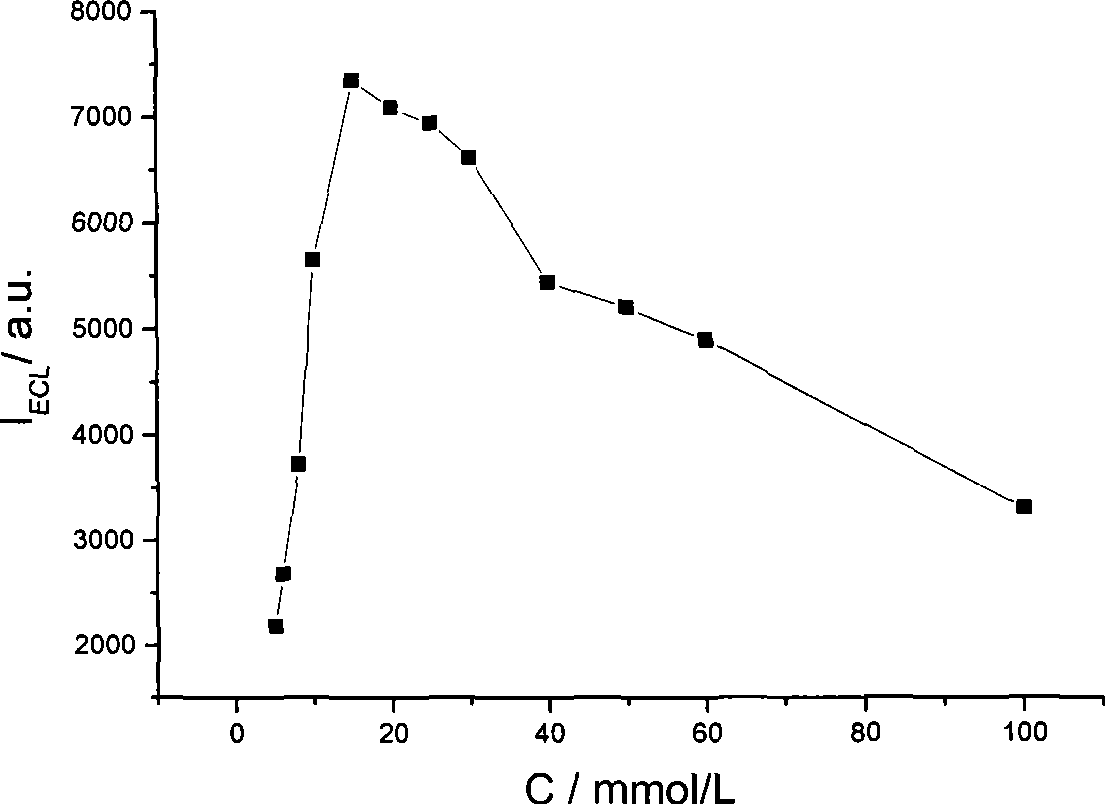
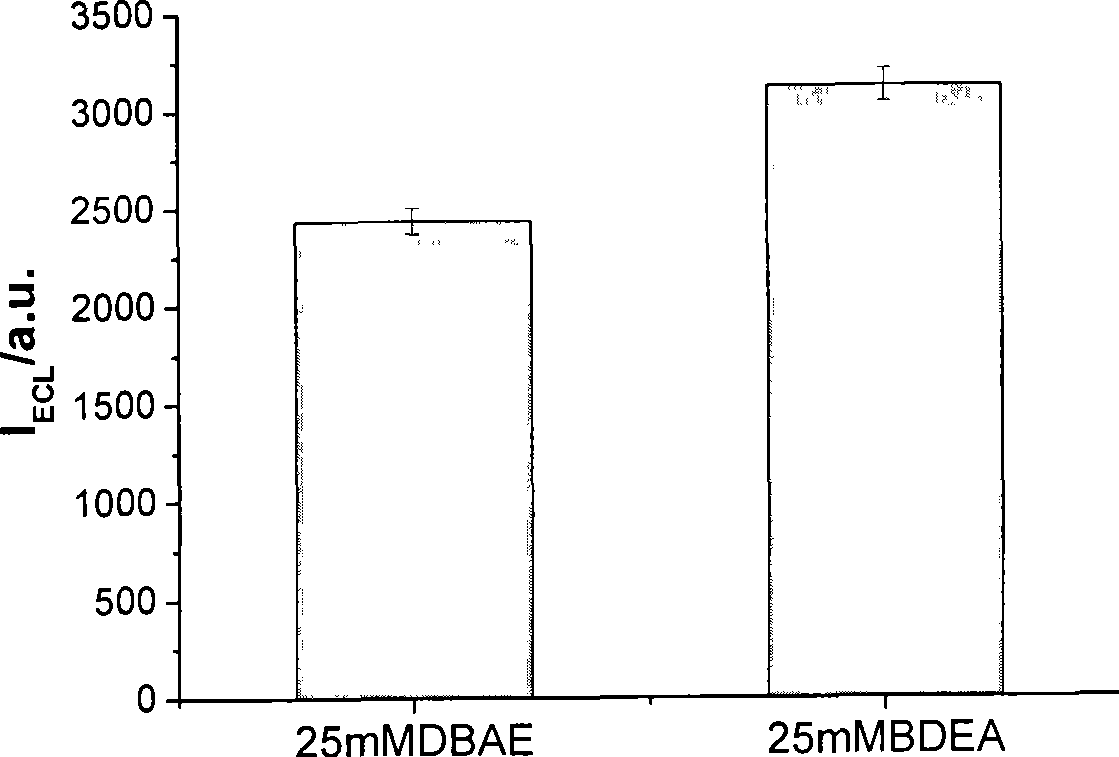
[0012] Embodiment 1: detection using an electrochemical workstation and a BPCL weak luminescence measuring instrument. The concentration of fixed n-butyldiethanolamine is 0.05mmol / L, the concentration of ruthenium pyridine is 1μmol / L, the buffer solution is 0.1mol / L phosphate with pH 7.5, and the gold electrode is used as the working electrode, and a step of 1.5V is applied At the same time, a luminescence detection voltage of 800V was applied to detect the luminescence signal of ruthenium pyridine, and the electrochemiluminescence detection was completed, and the average intensity of the luminescence signal obtained was 22.33.
Embodiment 2
[0013] Embodiment 2: using an electrochemical workstation and a BPCL weak luminescence measuring instrument to detect. The fixed n-butyldiethanolamine concentration is 15mmol / L, the buffer solution is 0.1mol / L phosphate at pH 7.5, add 4.0×10 -10 mol / L of the detected substance pyridine ruthenium, using a gold electrode as the working electrode, applying a step potential of 1.35V, and simultaneously applying a luminescence detection voltage of 700V, detecting the luminescence signal of pyridine ruthenium, completing the electrochemiluminescence detection, and obtaining the luminescence signal of pyridine ruthenium The average strength is 68.
Embodiment 3
[0014] Embodiment 3: detection using an electrochemical workstation and a BPCL weak luminescence measuring instrument. The concentration of fixed n-butyldiethanolamine is 1mmol / L, the concentration of ruthenium pyridinium to be tested is 1mmol / L, dissolved in 0.1mol / L phosphate buffer solution with pH 7.5, the gold electrode is used as the working electrode, and 1.5V is applied. Step potential, while applying a luminescence detection voltage of 500V, detects the luminescence signal of ruthenium pyridine, and completes the electrochemiluminescence detection. The average intensity of the luminescence signal obtained is 6751.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com