A cyclometallic iridium complex, its preparation method and its application as an electrochemiluminescence marker
A technology of iridium complexes and ring metals, applied in the field of biological analysis, can solve the problems of difficult luminescence wavelength, unavailable biomolecular markers, non-reactivity, etc., to overcome single luminescence color, good economic and social benefits, and huge market applications foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 The structural formula of the electrochemiluminescence marker used is as follows:
[0035]
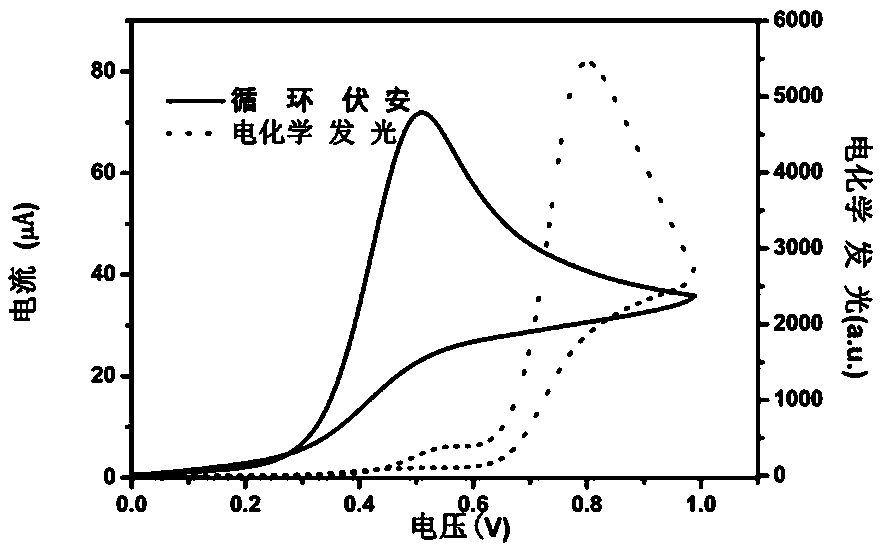
[0036] Using n-tripropylamine as a co-reactant, in PBS buffer solution, a 0 V-1 V-0 V cyclic voltammetry scan was applied on the electrode. As the scanning potential gradually increased, the electrode surface current gradually increased. - Tripropylamine is oxidized and the largest ECL signal is detected as figure 1 shown.
Embodiment 2
[0037] Example 2 The structural formula of the electrochemiluminescence marker used is as follows:
[0038]
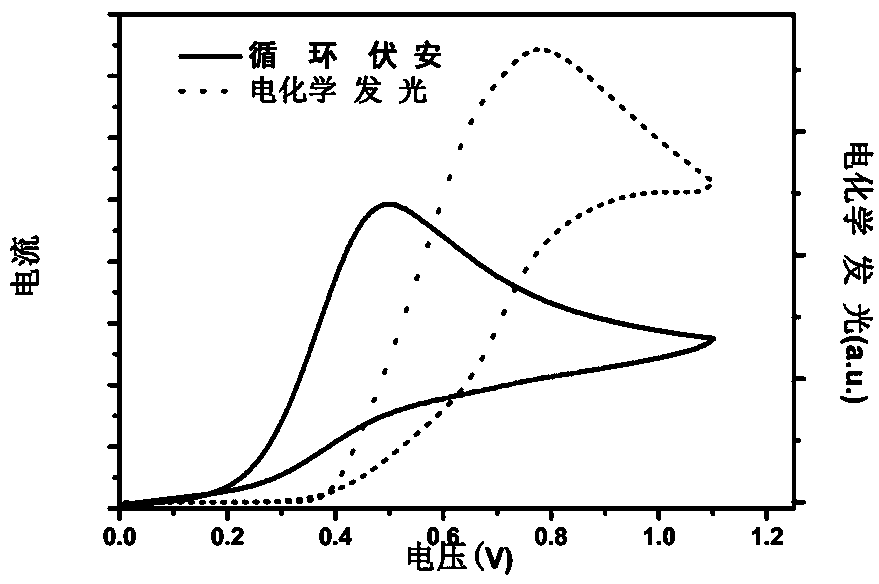
[0039] Using n-tripropylamine as a co-reactant, in PBS buffer solution, a 0 V-1 V-0 V cyclic voltammetry scan was applied on the electrode. As the scanning potential gradually increased, the electrode surface current gradually increased. -Tripropylamine was oxidized and the largest ECL signal was detected.
Embodiment 3
[0040] Example 3 The structural formula of the electrochemiluminescence marker used is as follows:
[0041]
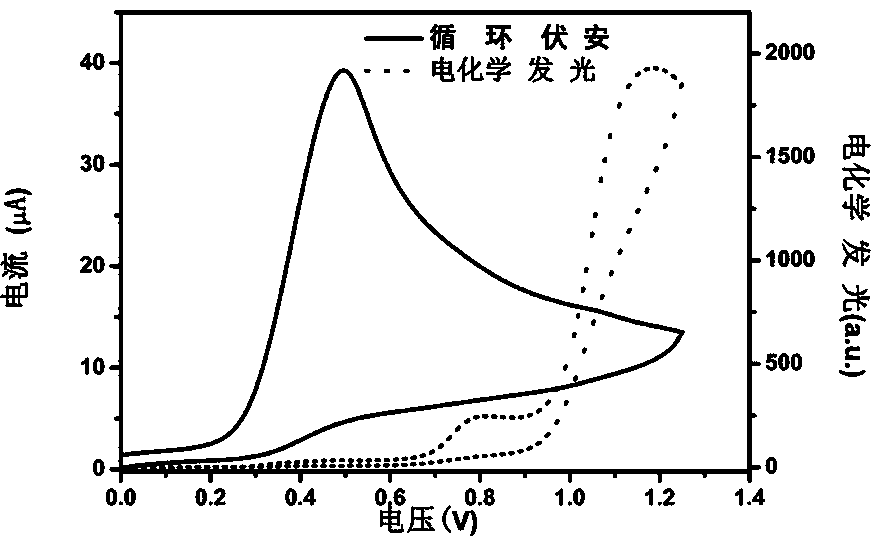
[0042] Using triethylamine as a co-reactant, in Tris buffer solution, a 0 V-1.2 V-0 V cyclic voltammetry scan was applied on the electrode. As the scanning potential gradually increased, the electrode surface current gradually increased. With the co-reaction The substance is oxidized, and the largest electrochemiluminescence signal is detected, as shown in the attached figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com