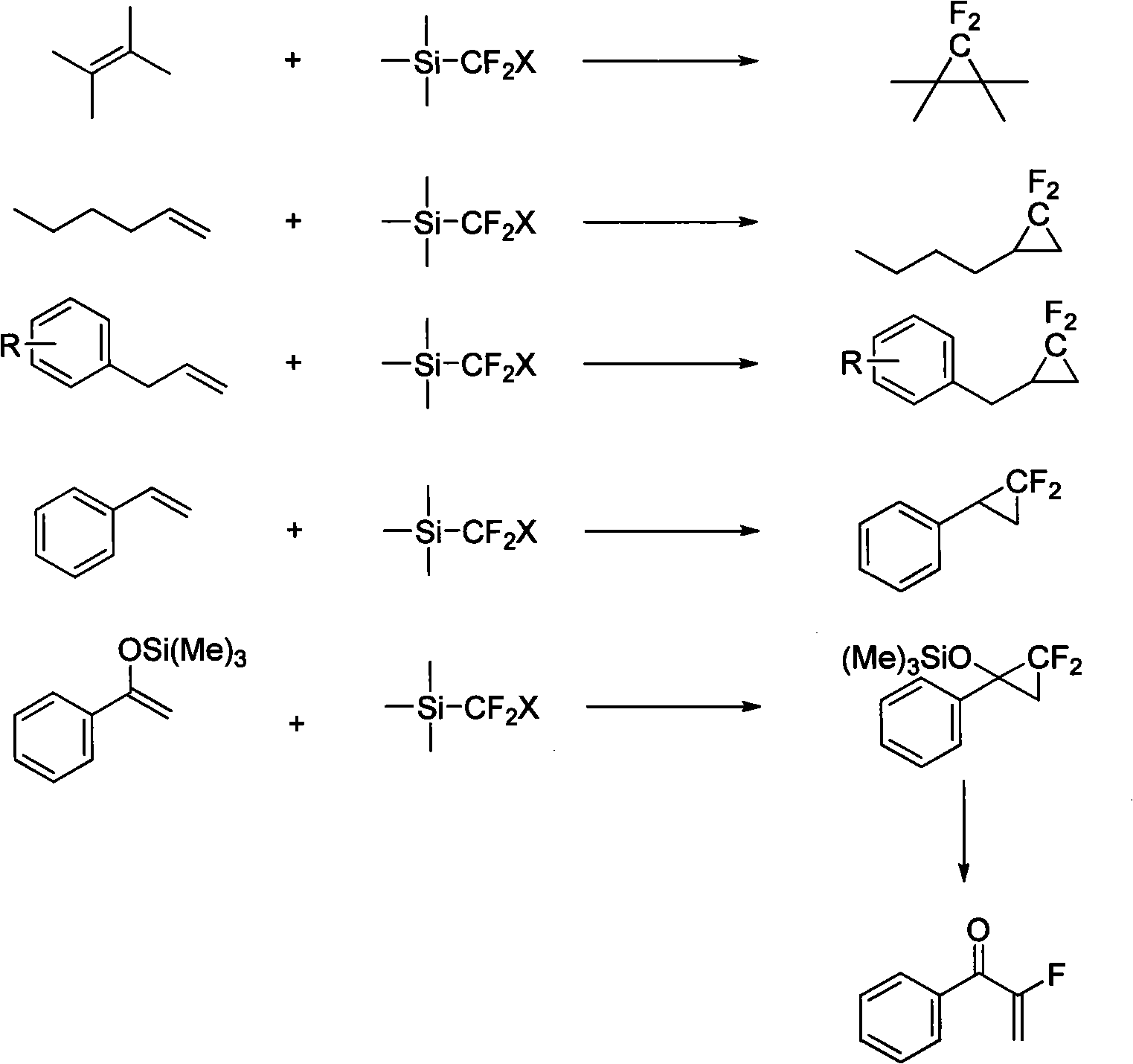
Method for preparing compounds containing difluoromethylene
A technology of difluoromethylene and halodifluoromethyltrialkylsilane, which is applied to the preparation of organic compounds, chemical instruments and methods, and the preparation of halogenated hydrocarbons, and can solve the problems of high toxicity, limited application, and complex synthesis and other problems, to achieve the effect of mild conditions, high universality and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Styrene (104mg, 1.0mmol), (Me) 3 SiCF 2 Cl (474mg, 3.0mmol) and 4ml THF (re-distilled) were added to the sealed tube with a syringe, tetrabutylammonium chloride (6mg, 0.02mmol) was quickly added to the reaction solution, the sealed tube was screwed tightly, and then Seal the tube and place it in an oil bath at 110° C., and stir for 4 hours to react. Produced product 126 mg, 82% yield.
Embodiment 2
[0022] Styrene (132mg, 1.0mmol), (Me) 3 SiCF 2 Cl (474mg, 3.0mmol) and 4ml THF (re-distilled) were added to the sealed tube with a syringe, tetrabutylammonium chloride (6mg, 0.02mmol) was quickly added to the reaction solution, the sealed tube was screwed tightly, and then Seal the tube and place it in an oil bath at 110° C., and stir for 4 hours to react. Produced product 104 mg, 57% yield.
Embodiment 3
[0024] Styrene (84mg, 1.0mmol), (Me) 3 SiCF 2 Cl (474mg, 3.0mmol) and 4ml THF (re-distilled) were added into the sealed tube with a syringe, tetrabutylammonium chloride (6mg, 0.02mmol) was quickly added to the reaction solution, the sealed tube was screwed tightly, and then Seal the tube and place it in an oil bath at 110° C., and stir for 4 hours to react. Produced product The yield of fluorine spectrum is 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com