Therapeutic agent for urinary tract disease
A technology for urinary incontinence and urination disorder, which is applied in the field of therapeutic drugs for urinary system diseases, and can solve problems such as non-existence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
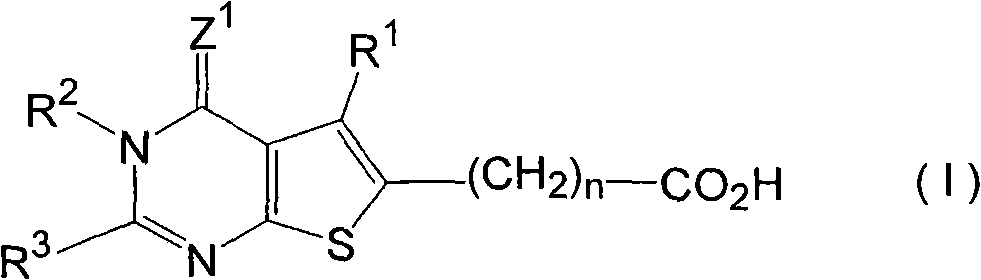
[0498] 5-Methyl-4-oxo-2-(thiophen-3-ylmethyl)-3,4-dihydrothieno[2,3-d]pyrimidine-6-carba acid (Compound number: A-1)
[0499]
[0500] 1-a): 5-methyl-4-oxo-2-(thiophen-3-ylmethyl)-3,4-dihydrothieno[2,3-d]pyrimidine -Synthesis of ethyl 6-formate
[0501] Add 515 mg of 5-amino-3-methylthiophene-2,4-dicarboxylated diethyl ester and 296 mg of 3-thiopheneacetonitrile to 8 mL of 4N hydrogen chloride in dioxane, and stir for 10 hours. After stirring, the reaction mixture was placed on ice and adjusted to pH 8-9 with 25% ammonia water. The precipitated crystals were collected by filtration, washed with water and hexane in sequence. The crude crystals were recrystallized from a mixture of N,N-dimethylformamide and cyclohexane to obtain 397 mg of the title compound.
[0502] 1 H-NMR (DMSO-d 6 )δ: 1.30 (3H, t, J = 7.1Hz), 2.81 (3H, s), 3.97 (2H, s), 4.30 (2H, q, J = 7.1Hz), 7.0-7.6 (3H, m), 12.74 (1H, br s)
[0503] MS (m / z): 334 (M + )
[0504] 1-b): 5-methyl-4-oxo-...
preparation example 2
[0510] 5-Methyl-4-oxo-2-(thiophen-2-ylmethyl)-3,4-dihydrothieno[2,3-d]pyrimidine-6-carba acid (Compound number: A-2)
[0511]
[0512] 1 H-NMR (DMSO-d 6 )δ: 2.79 (3H, s), 4.17 (2H, s), 6.9-7.5 (3H, m), 12.75 (1H, br s), 13.35 (1H, br s)
[0513] MS (m / z): 306 (M + )
preparation example 3
[0515] 2-(5-Chlorothiophen-2-ylmethyl)-5-methyl-4-oxo-3,4-dihydrothieno[2,3-d]pyrimidine -6-Formic acid (Compound number: A-3)
[0516]
[0517] 1 H-NMR (DMSO-d 6 )δ: 2.79 (3H, s), 4.13 (2H, s), 6.91 (1H, d, J = 3.9Hz), 6.98 (1H, d, J = 3.9Hz), 12.74 (1H, br s), 13.37 (1H, br s)
[0518] MS (m / z): 342 (M + +2), 340(M + )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com