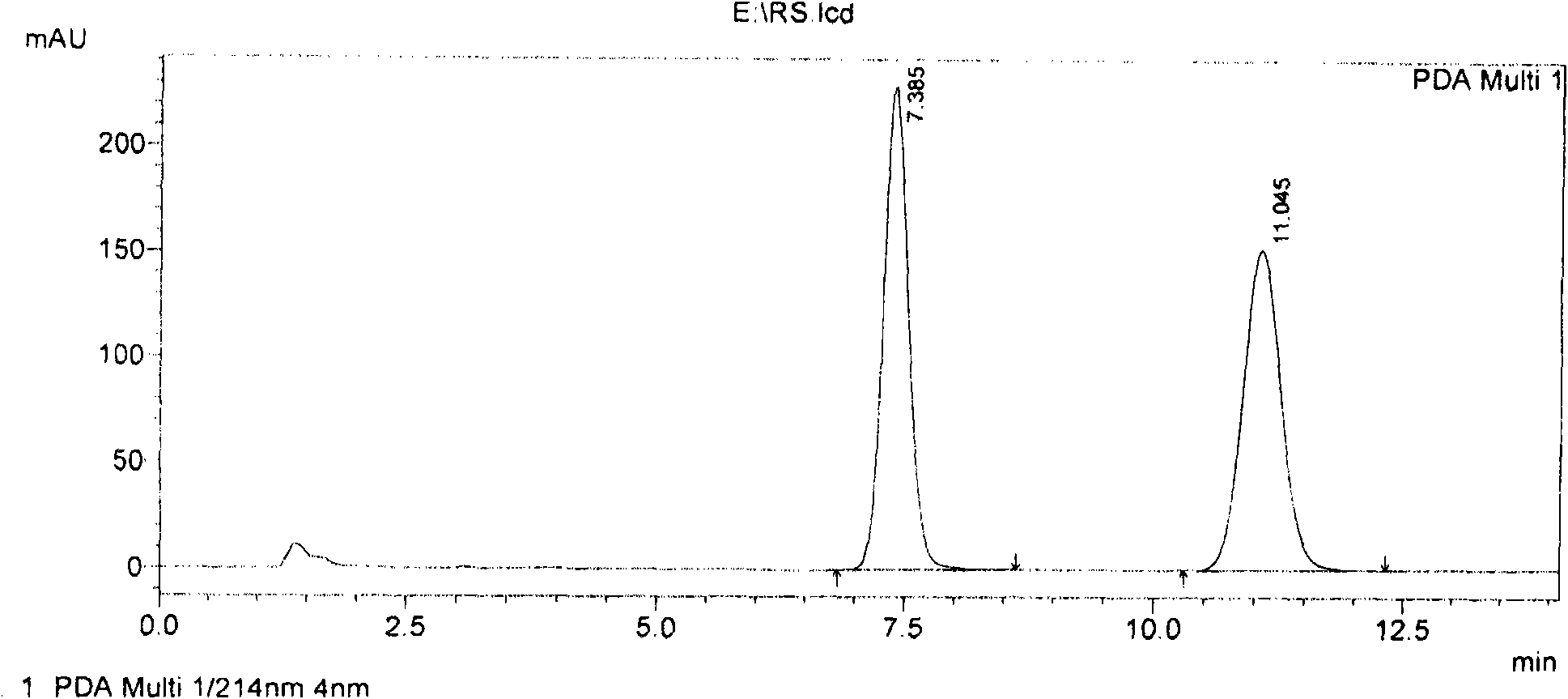
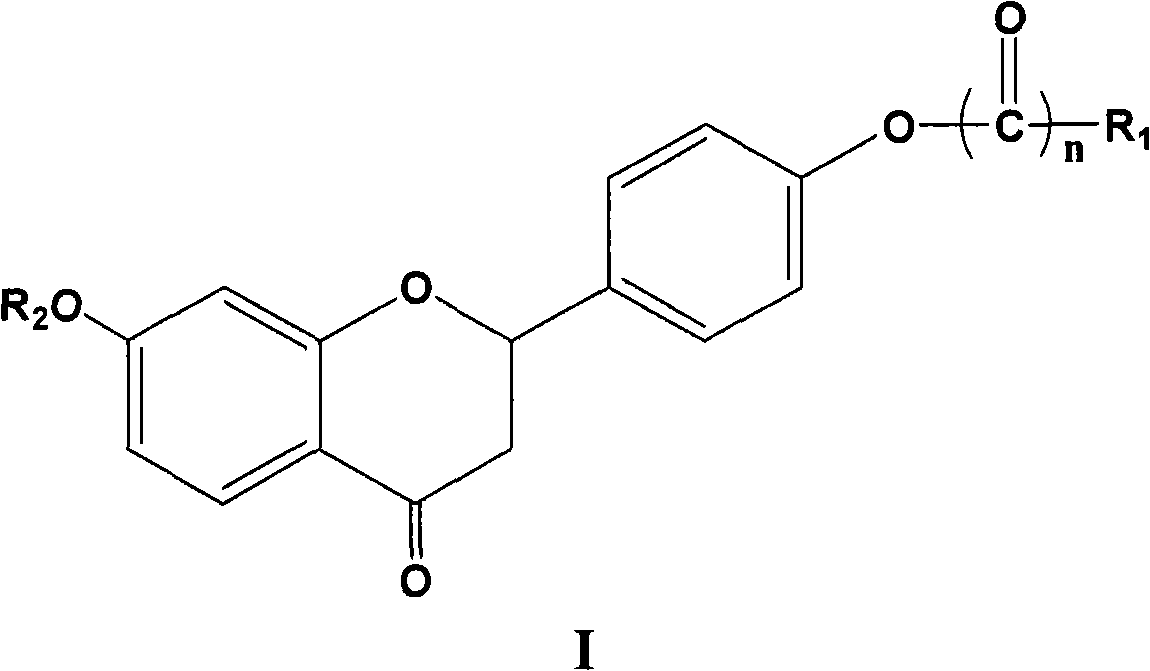
Glycyrrhizin derivatives and preparation and use thereof
A technology of drugs and compounds, applied in the field of pharmaceutical compositions containing them, can solve problems such as poor therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0069] All the documents cited in the present invention are incorporated herein by reference in their entirety, and if the meaning expressed in these documents is inconsistent with the present invention, the expression of the present invention shall prevail. In addition, various terms and phrases used in the present invention have common meanings known to those skilled in the art. Even so, the present invention still hopes to make a more detailed description and explanation of these terms and phrases here. The terms and phrases mentioned are as follows: If there is any inconsistency with the known meaning, the meaning expressed in the present invention shall prevail.
[0070] The term "halogen", "Hal" or "halo" refers to fluorine, chlorine, bromine, and iodine.
[0071] The terms "alkyl", "alkenyl" and "alkynyl" used in the present invention have the common meanings known in the art, which are straight-chain or branched hydrocarbon groups, such as but not limited to methyl, et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com