Data analysis for an implantable restriction device and a data logger
A technology of limiting devices and data, applied in data processing applications, patient-specific data, diagnostic records/measurements, etc., can solve problems such as inability to provide regulatory efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
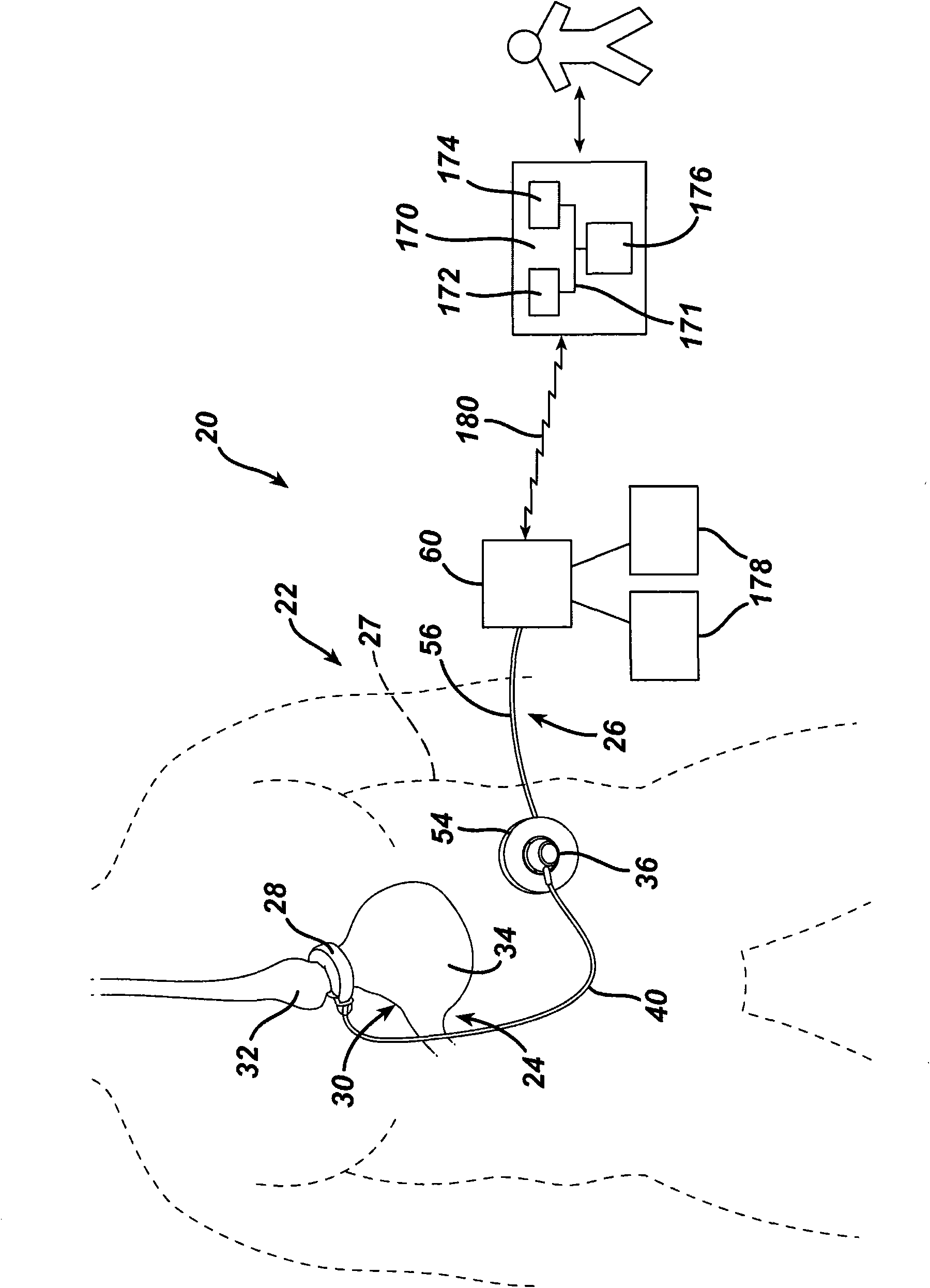
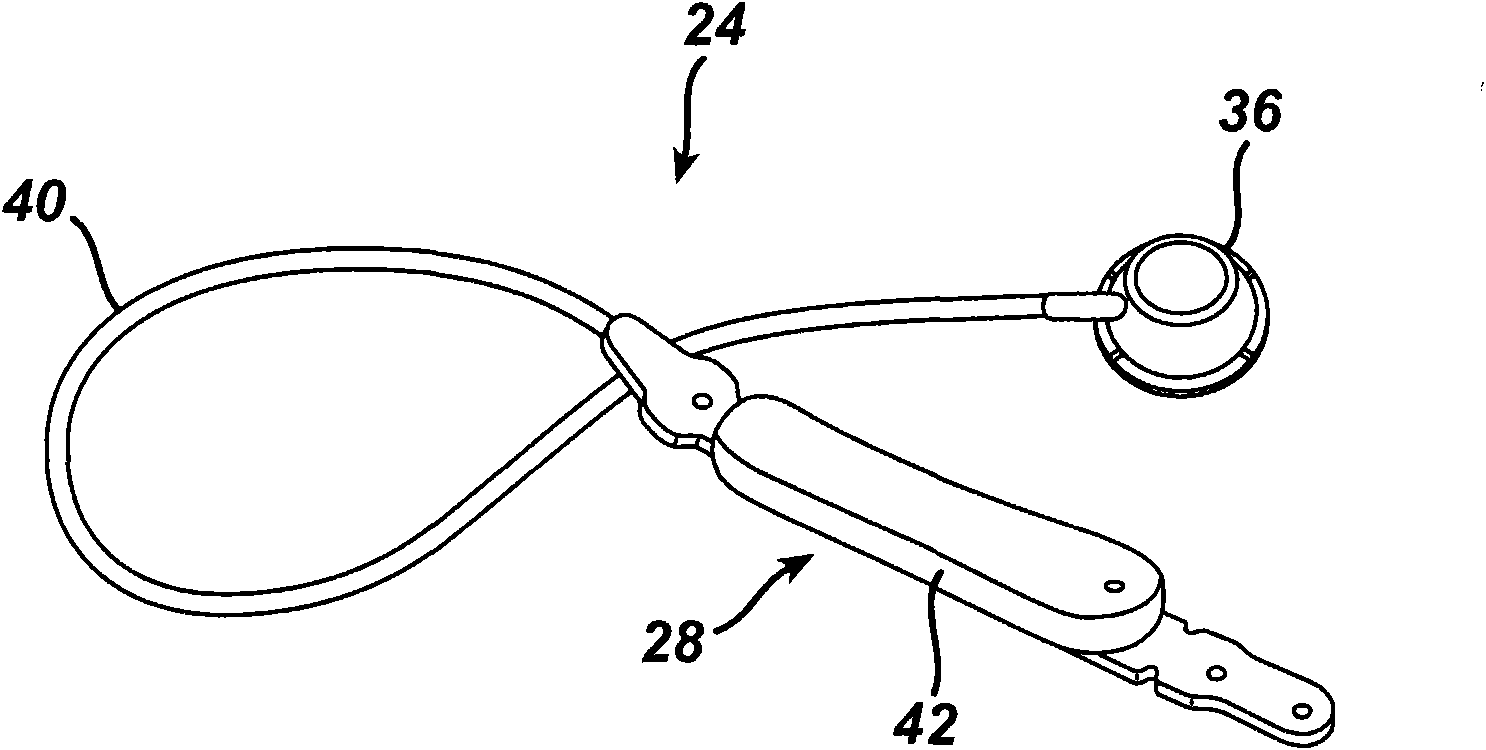
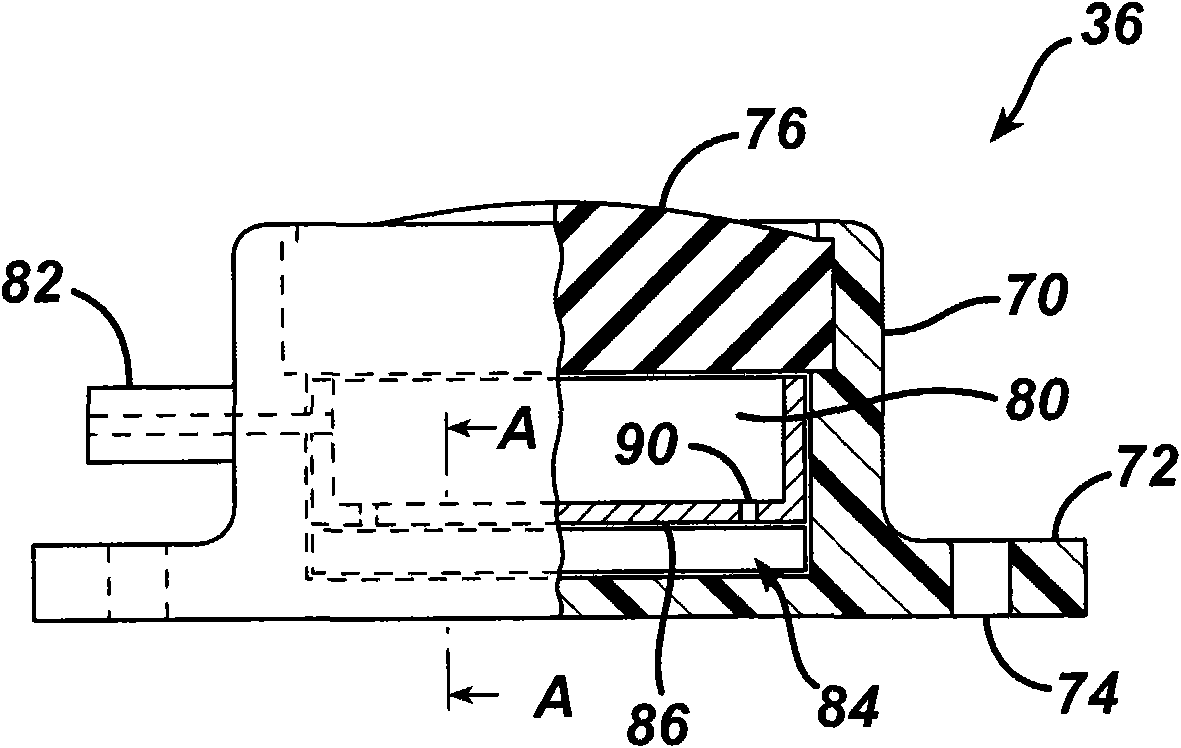
[0167] return Figure 4 , the output signals from the differential amplifiers 102 , 104 are provided to the microcontroller 106 . Microcontroller 106 is integrated into circuit board 110 inside housing 94 . The temperature sensor 112 measures the temperature within the injection port 36 and inputs the temperature signal to the microcontroller 106 . Microcontroller 106 uses the temperature signal from sensor 112 to compensate for body temperature variations and residual temperature errors not accounted for by strain gauge 98 . Compensating the pressure measurement signal for changes in body temperature increases the accuracy of the pressure sensor 84 . Additionally, a TET / telemetry coil 114 is placed within the housing 94 . Coil 114 is connected to capacitor 116 to form a tuned tank circuit for receiving power from local unit 60 for communicating physiological data, including measured fluid pressure, to local unit 60 . Figures 3 to 5 One exemplary embodiment for measuring ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com