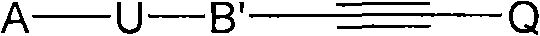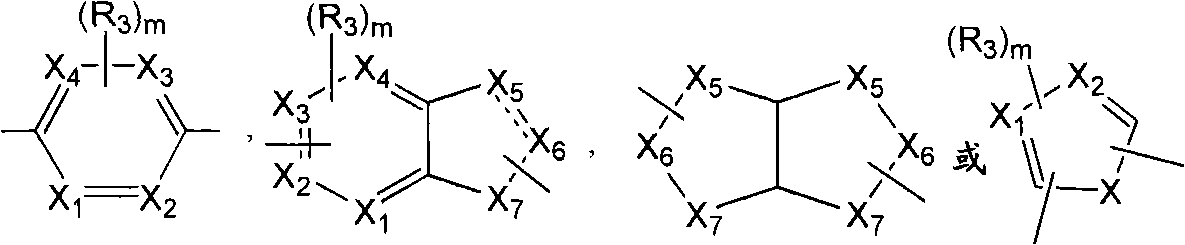Acetylene derivatives as stearoyl coa desaturase inhibitors
A solvate and compound technology, applied in the field of stearoyl CoA desaturase inhibitors, can solve problems such as weak non-specific SCD1 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
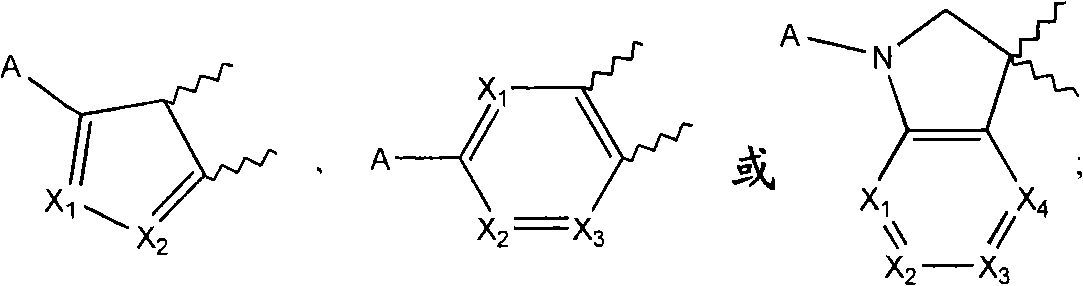
[0058] According to a preferred embodiment of the present invention, the SCD1 inhibitor is selected from
[0059]
[0060] Formula II Formula III
[0061]
[0062] Formula IV Formula V
[0063]
[0064] Formula VI Formula VIII
[0065]
[0066] Formula VII
[0067] or a pharmaceutically acceptable salt, solvate, stereoisomer, prodrug or N-oxide thereof, wherein:
[0068] R' is selected from substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted cycloalkylalkyl, substituted or unsubstituted cycloalkenyl, substituted or unsubstituted cycloalkenylalkyl, substituted or unsubstituted aryl, substituted or unsubstituted arylalkyl, substituted or unsubstituted heteroaryl, substituted or unsubstituted Heteroarylalkyl, substituted or unsubstituted heterocycle and substituted or unsubstituted heterocyclylalkyl;
[0069] R is selected from hydroge...
Embodiment 1
[0498] 4-[5-(3-Hydroxy-1-propynyl)-2-pyridyl]piperazin-1-yl-2-trifluoromethyl phenyl ketone
[0499]
[0500] The product is obtained by the presence of PdCl 2 (PPh 3 ) 2 (20mg, 0.0284mmol) and CuI (16mg, 0.084mmol) in the case of intermediate 1 (1.1g, 2.827mmol) and prop-1-yn-1-ol (317mg, 5.655mmol) in triethylamine in Preparation was carried out by Sonogashira coupling reaction under nitrogen for 18 hours. The crude product obtained after extractive workup with chloroform was purified by column chromatography on silica gel, eluting with 30% EtOAc in chloroform, to give 270 mg of the product as an off-white solid; IR (KBr) 3298, 2851, 2240, 1626, 1497, 1242, 1010, 769cm -1 ; 1 H NMR (300MHz, CDCl 3 )δ1.81(br s, 1H, can be compared with D 2 O exchange), 3.28 (br s, 2H), 3.54-3.68 (m, 4H), 3.88-3.95 (m, 2H), 4.48 (s, 2H), 6.58 (d, J=8.4Hz, 1H), 7.36 (d, J=6.9Hz, 1H), 7.52-7.62(m, 3H), 7.74(d, J=7.2Hz, 1H), 8.26(s, 1H); ESI-MS (m / z) 390.30(M +H) + .
Embodiment 2
[0502] 4-[5-(3-Hydroxy-1-propynyl)-2-pyridyl]piperazin-1-yl-2,5-dichlorophenyl ketone
[0503]
[0504] Prepared by Sonogashira coupling of intermediate 3 with prop-1-yn-1-ol to give the product as a white solid; IR (KBr) 3351, 2846, 2237, 1628, 1495, 1239, 1012, 822cm -1 ; 1 H NMR (300MHz, CDCl 3 )δ1.71(t, J=6.3Hz, 1H, can be compared with D 2 O exchange), 3.30-3.39 (m, 2H), 3.60-3.69 (m, 4H), 3.85-3.96 (m, 2H), 4.49 (d, J=6.0Hz, 2H), 6.59 (d, J=9.0 Hz, 1H), 7.31-7.35 (m, 3H), 7.53 (d, J=8.7Hz, 1H), 8.27 (s, 1H); ESI-MS (m / z) 390.61 [100%, (M+H ) + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com