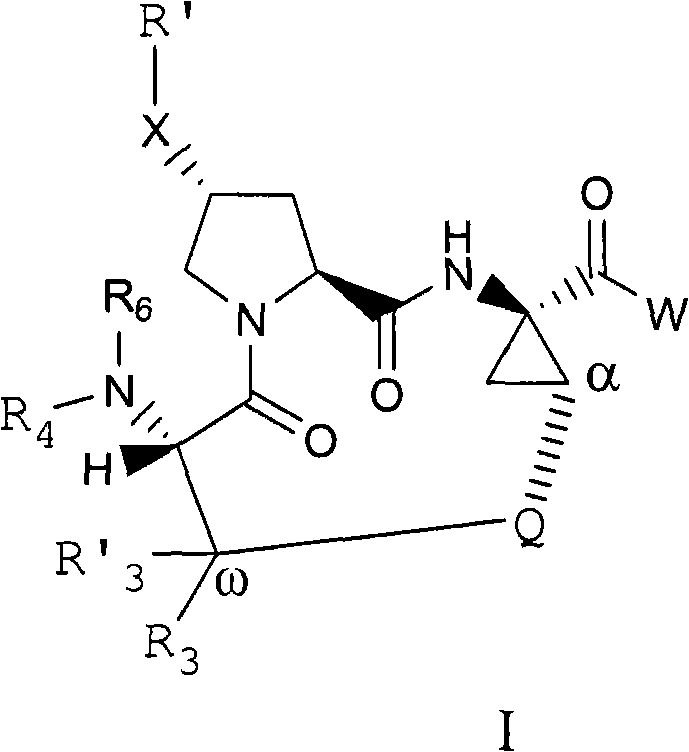
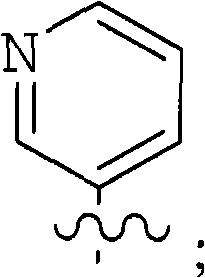
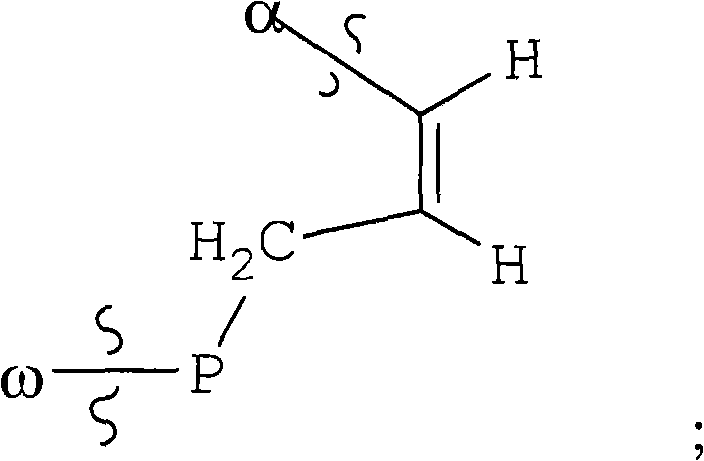
Inhibitors of hepatitis c virus
An alkyl and cycloalkyl technology, applied in the field of antiviral compounds, can solve the problem that the viral load does not continue to decrease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0177] Racemic ethyl (1R,2S) / (1S,2R)-1-amino-2-vinylcyclopropanecarboxylate hydrochloride Preparation (Method A and Method B)
[0178]
[0179] The compound was racemized by each of the following methods A and B.
[0180] Method A
[0181] Preparation of N-benzyl imine of ethyl glycine
[0182]
[0183] Glycine ethyl ester hydrochloride (303.8 g, 2.16 mol) was suspended in tert-butyl methyl ether (1.6 L). Benzaldehyde (231 g, 2.16 mol) and anhydrous sodium sulfate (154.6 g, 1.09 mol) were added and the mixture was cooled to 0°C using an ice water bath. Triethylamine (455 mL, 3.26 mol) was added dropwise over 30 minutes, and the mixture was stirred at room temperature for 48 hours. The reaction was then quenched by adding ice-cold water (1 L) and the organic layer was separated. The aqueous phase was extracted with tert-butyl methyl ether (0.5 L), and the combined organic phases were washed with a mixture of saturated aqueous NaHCO3 (1 L) and brine (1 L). The s...
Embodiment 2
[0195] Resolution of N-Boc-(1R, 2S) / (1S, 2R)-1-amino-2-vinylcyclopropanecarboxylic acid ethyl ester
[0196]
[0197] Racemates: (1R, 2S) and (1S, 2R)
[0198] 1:1 mixture of
[0199] Split A
[0200] To an aqueous solution of sodium phosphate buffer (0.1 M, 4.25 liters (“L”), pH 8) in a 12-liter jacketed reactor maintained at 39° C. and stirred at 300 rpm was added 511 grams of Alcalase 2.4 L (approx. 425 ml) (Novozymes North America Inc.). When the temperature of the mixture reached 39°C, the pH was adjusted to 8.0 by adding 50% NaOH in water. A solution of racemic ethyl N-Boc-(1R,2S) / (1S,2R)-1-amino-2-vinylcyclopropanecarboxylate (85 g) in 850 mL DMSO was then added over 40 minutes. The reaction temperature was then maintained at 40°C for 24.5 hours, during which time the pH of the mixture was adjusted to 8.0 with 50% NaOH in water at the 1.5 and 19.5 hour time points. After 24.5 hours, the enantiomeric excess of the ester was measured to be 97.2%, the reaction ...
Embodiment 3
[0247] Step 1: Preparation of ethyl 1(R)-amino-2(S)-vinylcyclopropanecarboxylate hydrochloride
[0248]
[0249] Ethyl 1(R)-tert-butoxycarbonylamino-2(S)-vinylcyclopropanecarboxylate (8.5 g, 33.3 mmol) was washed with 200 mL of 4N HCl / dioxane ( Aldrich) was stirred at room temperature for 3 hours. The solvent was removed under reduced pressure keeping the temperature below 40 °C. This yielded 6.57 g (-100%) of ethyl 1(R)-amino-2(S)-vinylcyclopropanecarboxylate hydrochloride as a light brown (tan) solid. 1H NMR (300MHz, CD 3 OD) δ1.31(t, J=7.0Hz, 3H), 1.69-1.82(m, 2H), 2.38(q, J=8.8Hz, 1H), 4.29(q, J=7.0Hz, 2H), 5.22 (d, J=10.3Hz, 1H), 5.40 (d, J=17.2Hz, 1H), 5.69-5.81 (m, 1H). MS m / z 156 (M++1).
[0250] Step 2: 1(R)-[1-tert-butoxycarbonyl-4(R)-hydroxypyrrolidine-2(S)-carboxamido Preparation of ethyl (carboxamido)]-2(S)-vinylcyclopropanecarboxylate
[0251]
[0252] With N-methylmorpholine (9.3 mL, 84.7 mmol), HATU (19.5 g, 51.3 mmol) and ethyl 1(R)-amino-2(S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com