Substituted acetophenones useful as PDE4 inhibitors
A kind of substituent and representative technology, applied in the field of preparing medicine, can solve the problems such as limiting the clinical application of theophylline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
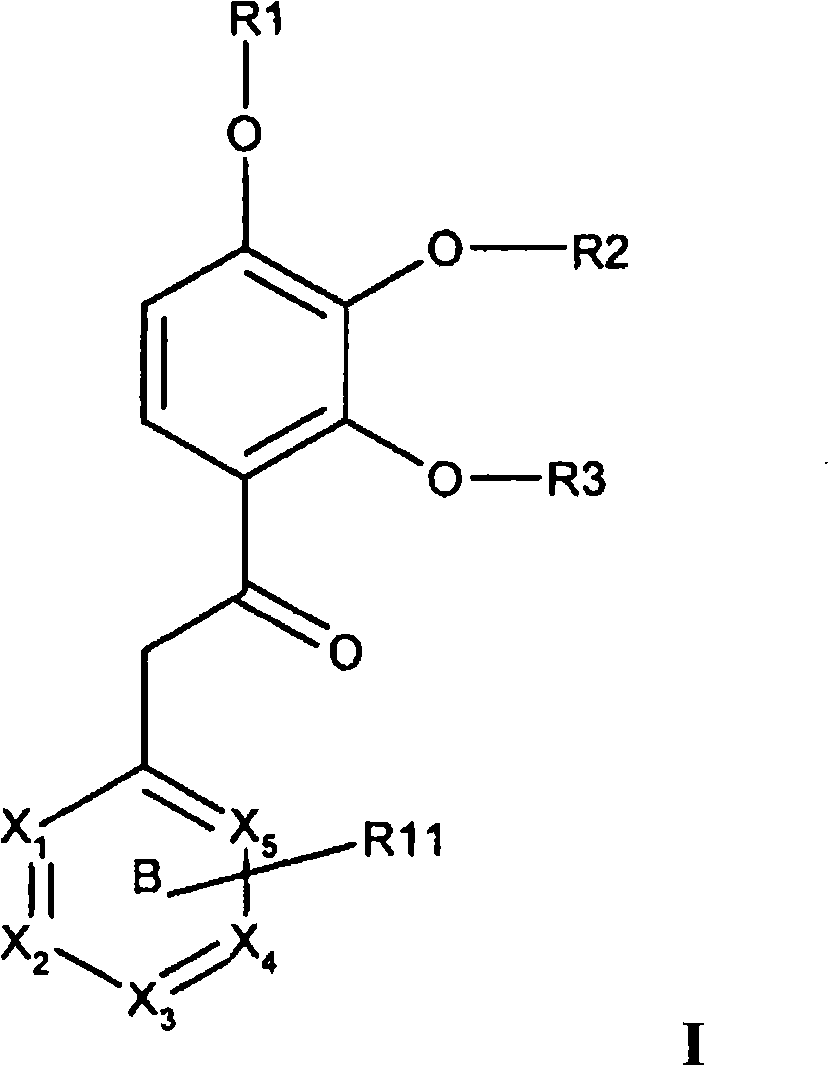
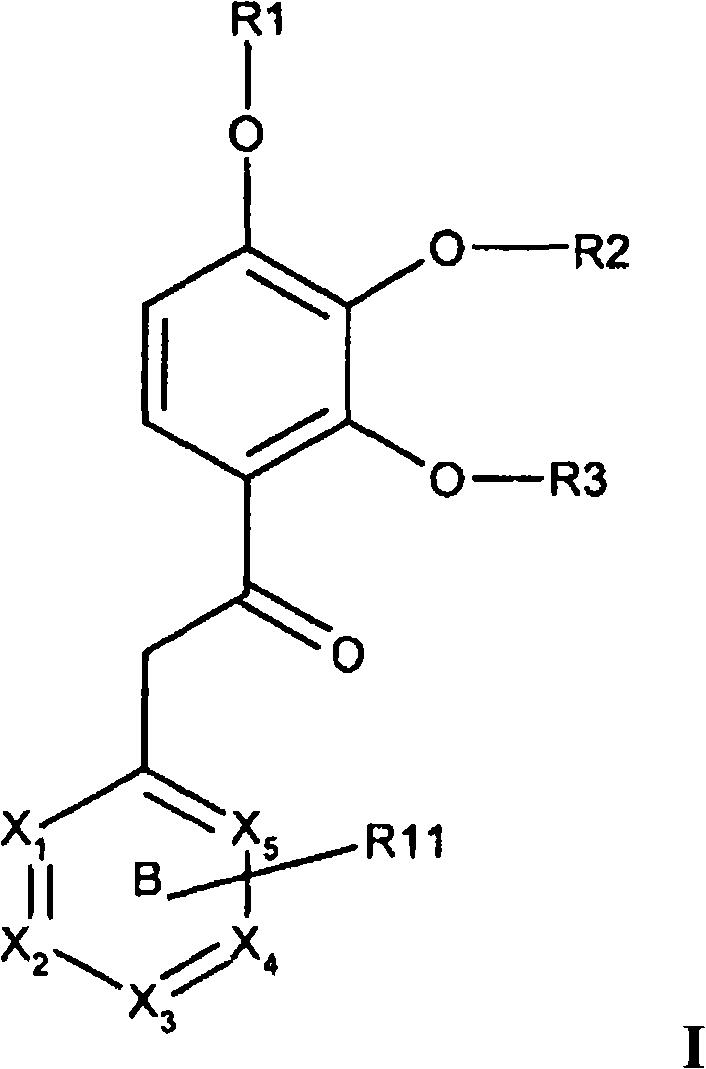
[0064] In one or more embodiments of the present invention, ring B represents pyridyl, pyrazinyl, quinolinyl, pyrimidinyl or pyridazinyl, which is optionally selected from one or more, the same or different Substituted by the following substituents: fluorine, chlorine, bromine, cyano, methoxy, -NH 2 or C 1-4 Amino.
[0065] In one or more embodiments of the invention, optionally R 11 The substituted ring B represents 2-(6-chloro-pyrazinyl), 2-pyrazinyl, 4-(3-bromo-pyridyl), 4-(3,5-dibromo-pyridyl), 4 -(6-chloro-pyrimidinyl), 2-(4-chloro-pyridyl), 3-(2-chloro-pyridyl), 4-(2-methoxy-pyridyl), 4-(2- cyano-pyridyl), 3-pyridazinyl, 4-(2-tert-butylamino-3,5-dichloro-pyridyl), 4-(2-amino-3,5-dichloro-pyridyl ), 4-(3,5-dichloro-pyridyl), 2-(3-bromo-pyrazinyl), 4-pyridyl, 4-quinolyl or 4-(3,5-dichloro-1 -oxy-pyridyl).
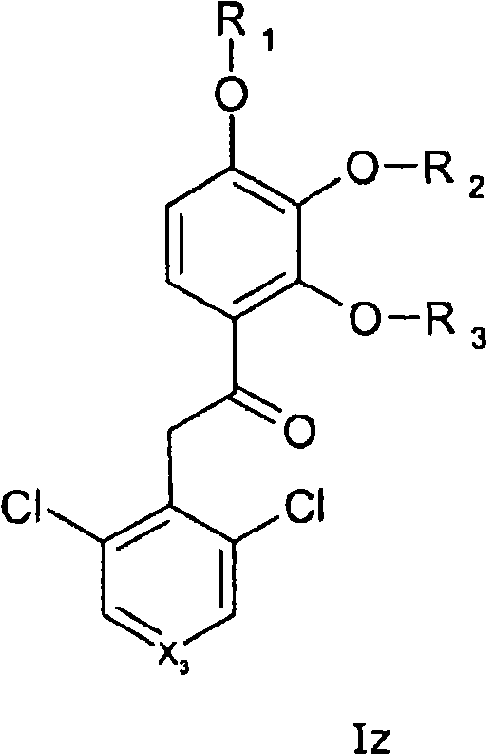
[0066] In one or more embodiments of the invention, formula I represents the general formula Iz,
[0067]
[0068] where X 3 represents -CH- or N, and where ...
Embodiment 1
[0409] Example 1 (compound 101)
[0410] 2-(3,5-Dichloro-pyridin-4-yl)-1-(2-hydroxy-3,4-dimethoxy-phenyl)-ethanone
[0411]
[0412] 2-(3,5-Dichloro-pyridin-4-yl)-1-(2,3,4-trimethoxy-phenyl)-ethanone (20 g, 56 mmol) was dissolved in DCM (75 mL). Add BCl slowly over 2 hours at room temperature 3 (95 mL of 1M in DCM). Water (50 mL) was added slowly, followed by EtOH (200 mL). The white precipitate was filtered and recrystallized from EtOH. 2-(3,5-Dichloro-pyridin-4-yl)-1-(2-hydroxy-3,4-dimethoxy-phenyl)-ethanone was obtained as a white solid. 1 H NMR (CDCl 3 )δ=11.92(1H,s), 8.54(2H,s), 7.68(1H,d), 6.60(1H,d), 4.67(2H,s), 3.98(3H,s), 3.90(3H,s ). Yield 14.2 g (74%)
[0413] Preparation 3 (Compound 503)
[0414] 2-(3,5-Dichloro-pyridin-4-yl)-1-(2,3-dihydroxy-4-methoxy-phenyl)-ethanone
[0415]
[0416] 2-(3,5-Dichloro-pyridin-4-yl)-1-(2,3,4-trimethoxy-phenyl)-ethanone (1.25 g , 3.5 mmol) was dissolved in AcOH (100%, 9.25 mL). HI (55-58%, 4.55 mL) was added and...
Embodiment 2
[0421] Example 2 (compound 102)
[0422] 2-(3,5-dichloro-pyridin-4-yl)-1-(3-hydroxy-2,4-dimethoxy-phenyl)-ethanone LC / MS (method B): (m / z) 342.1 (MH+); RT = 3.34 min; Purity (UV) = 100%; Alkyl halide: methyl iodide
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com