Nicotinylmethylamide freeze-dried powder preparation for injection and preparation method thereof
A technology of freeze-dried powder injection and oxymethyleneamine, which is applied in the field of medicine, can solve the problems of no oxymethonia injections on the market and a single choleretic dosage form.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
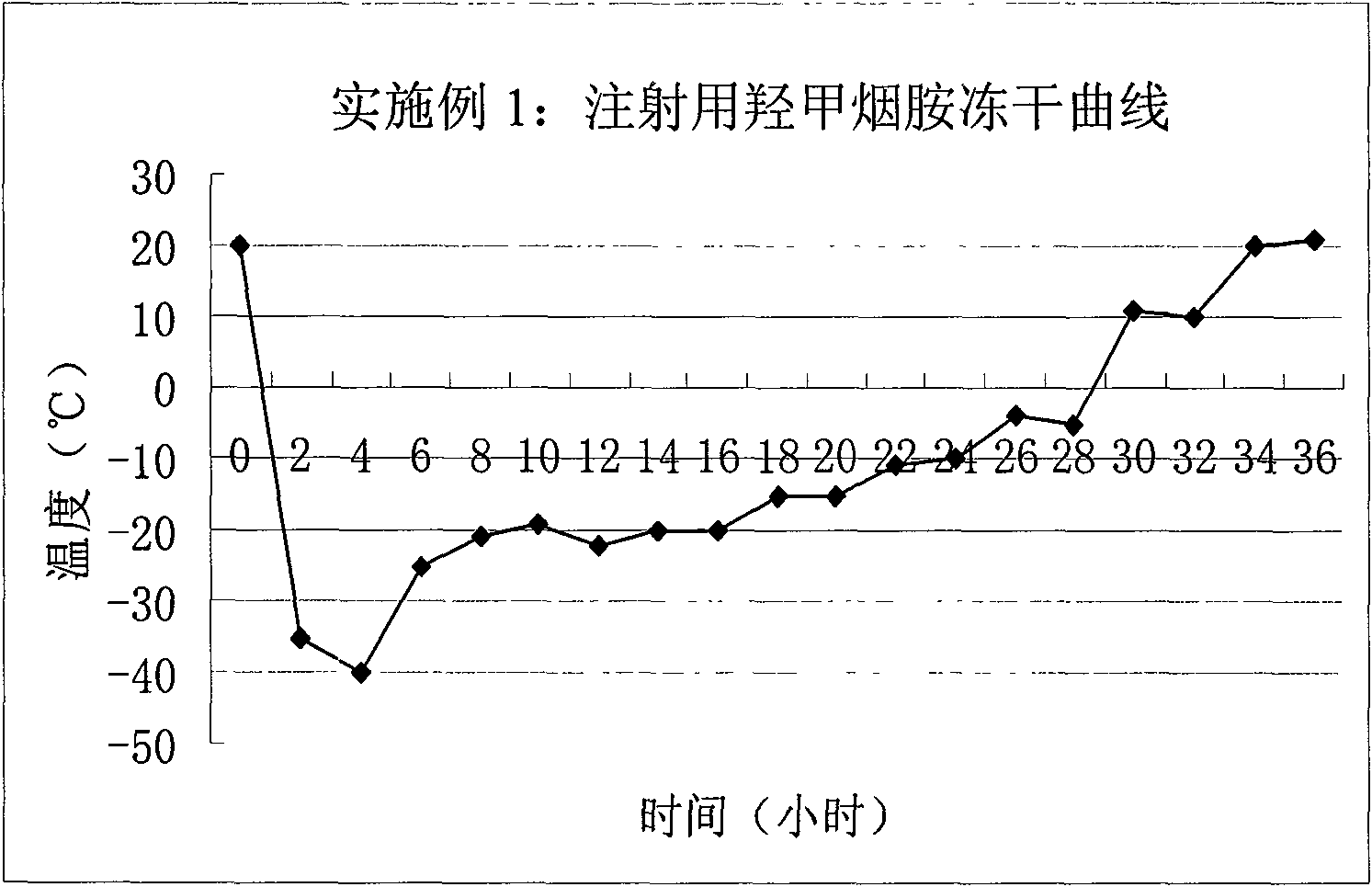
Embodiment 1
[0037] prescription
[0038] Oxymethonamide 250g
[0039] Mannitol 25g
[0040]
[0041] Add water for injection to 5L
[0042] Preparation Process
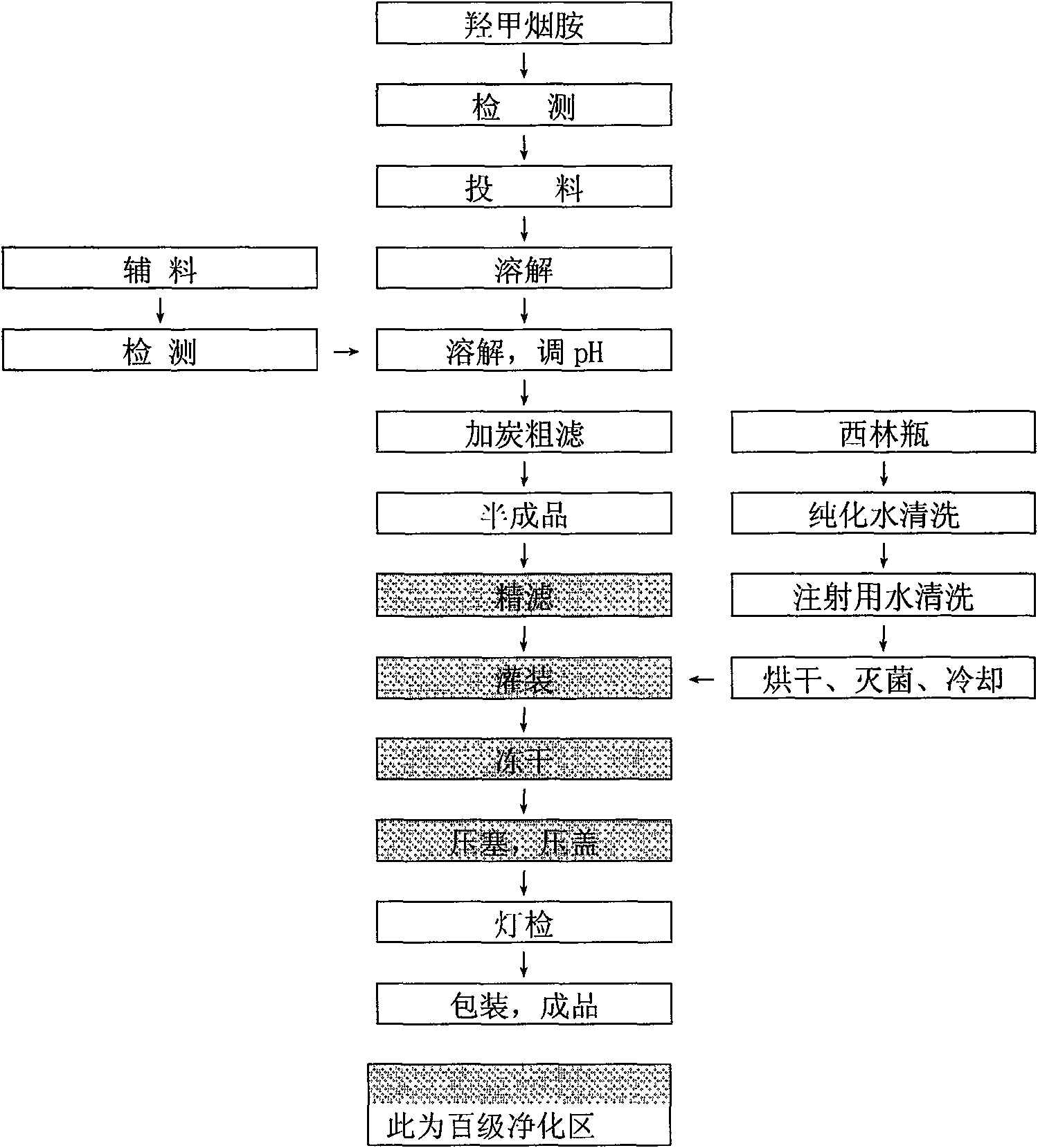
[0043] The freeze-dried preparation of oxymethyleneamide was prepared under the aseptic condition of 100-grade purification.
[0044] (1) Weigh 250 g of oxymethyleneamine into the mixing tank, add 4000 ml of water for injection into the mixing tank, stir to make the main ingredient completely dissolve;
[0045] (2) Weigh 25 g of mannitol into the above-mentioned mixing tank, stir to dissolve completely, add water for injection to 5000 ml, measure the pH value of the solution to 6.31, within the range of 6-8, do not need to adjust, stir evenly;
[0046] (3) Add 0.1% activated carbon for needles of the total volume of the above solution, stir for 30 minutes, filter the solution under reduced pressure with a titanium rod to remove carbon, and then fine filter with a 0.22 μm microporous membrane to obtain...
Embodiment 2
[0055] prescription
[0056] Oxymethonamide 250g
[0057] Dextran 50g
[0058]
[0059] Add water for injection to 5L
[0060] Preparation Process
[0061] The freeze-dried preparation of oxymethyleneamide was prepared under the aseptic condition of 100-grade purification.
[0062] (1) Weigh 250 g of oxymethyleneamine into the mixing tank, add 4000 ml of water for injection into the mixing tank, stir to make the main ingredient completely dissolve;
[0063] (2) Weigh 50 g of dextran into the above-mentioned mixing tank, stir to dissolve completely, add water for injection to 5000 ml, measure the pH value of the solution to 6.29, within the range of 6-8, do not need to adjust, stir evenly;
[0064] (3) Add 0.2% activated carbon for needles of the total volume of the above solution, stir for 30 minutes, filter the solution under reduced pressure with a titanium rod to remove carbon, and then fine filter with a 0.22 μm microporous membrane to obtain ...
Embodiment 3
[0069] prescription
[0070] Oxymethonamide 300g
[0071]
[0072] Add water for injection to 5L
[0073] Preparation Process
[0074] The freeze-dried preparation of oxymethyleneamide was prepared under the aseptic condition of 100-grade purification.
[0075] (1) Weigh 300 g of oxymethyleneamine into the mixing tank, add 4500 ml of water for injection into the mixing tank, and stir to completely dissolve the main ingredient;
[0076] (2) Add water for injection to 5000ml, measure the pH value of the solution to 7.42, within the range of 6-8, no need to adjust, stir evenly;
[0077] (3) Add 0.2% activated carbon for needles of the total volume of the above solution, stir for 30 minutes, filter the solution under reduced pressure with a titanium rod to remove carbon, and then fine filter with a 0.22 μm microporous membrane to obtain a oxymethyleneamine solution;
[0078] (4), get the oxymethyleneamine solution to measure the pH value, measure the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com