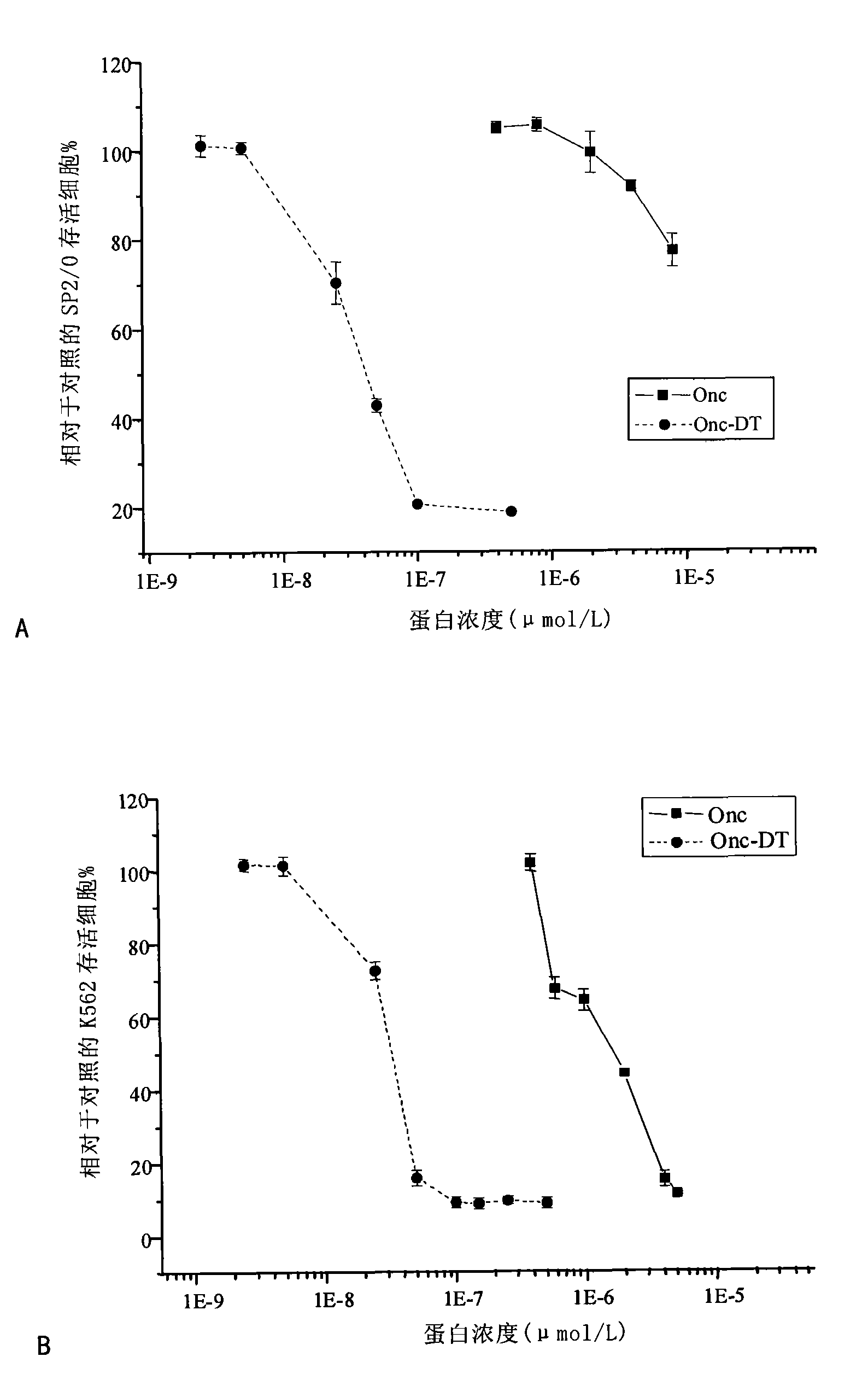
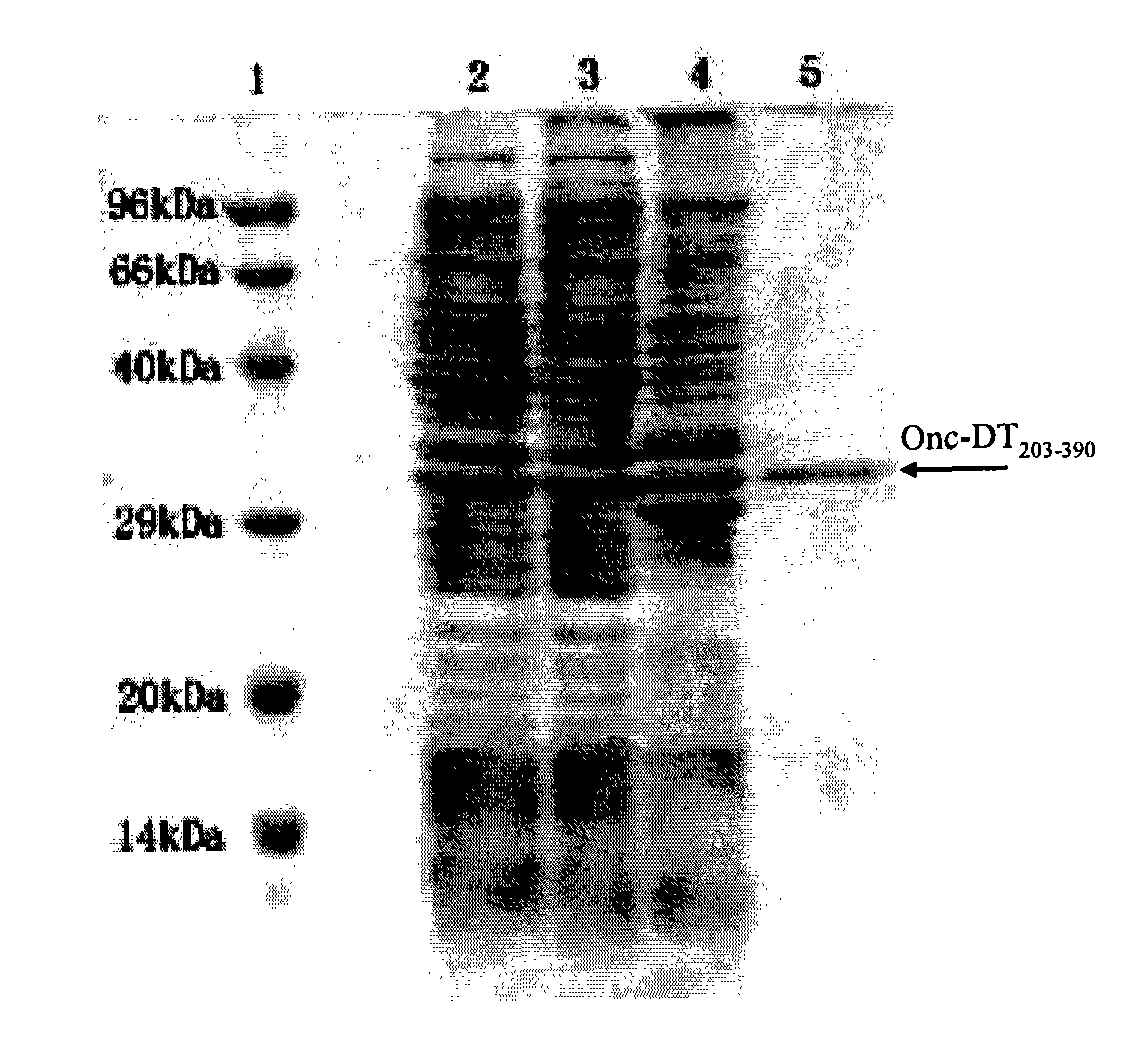Fusion protein of ribonuclease and toxalbumin membrane transposition structural domain, preparation and application thereof
A technology of ribonuclease and fusion protein, which is applied in the field of DNA recombination technology and biomedicine, and can solve the problems of limiting the scope of application of Onc and different sensitivities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
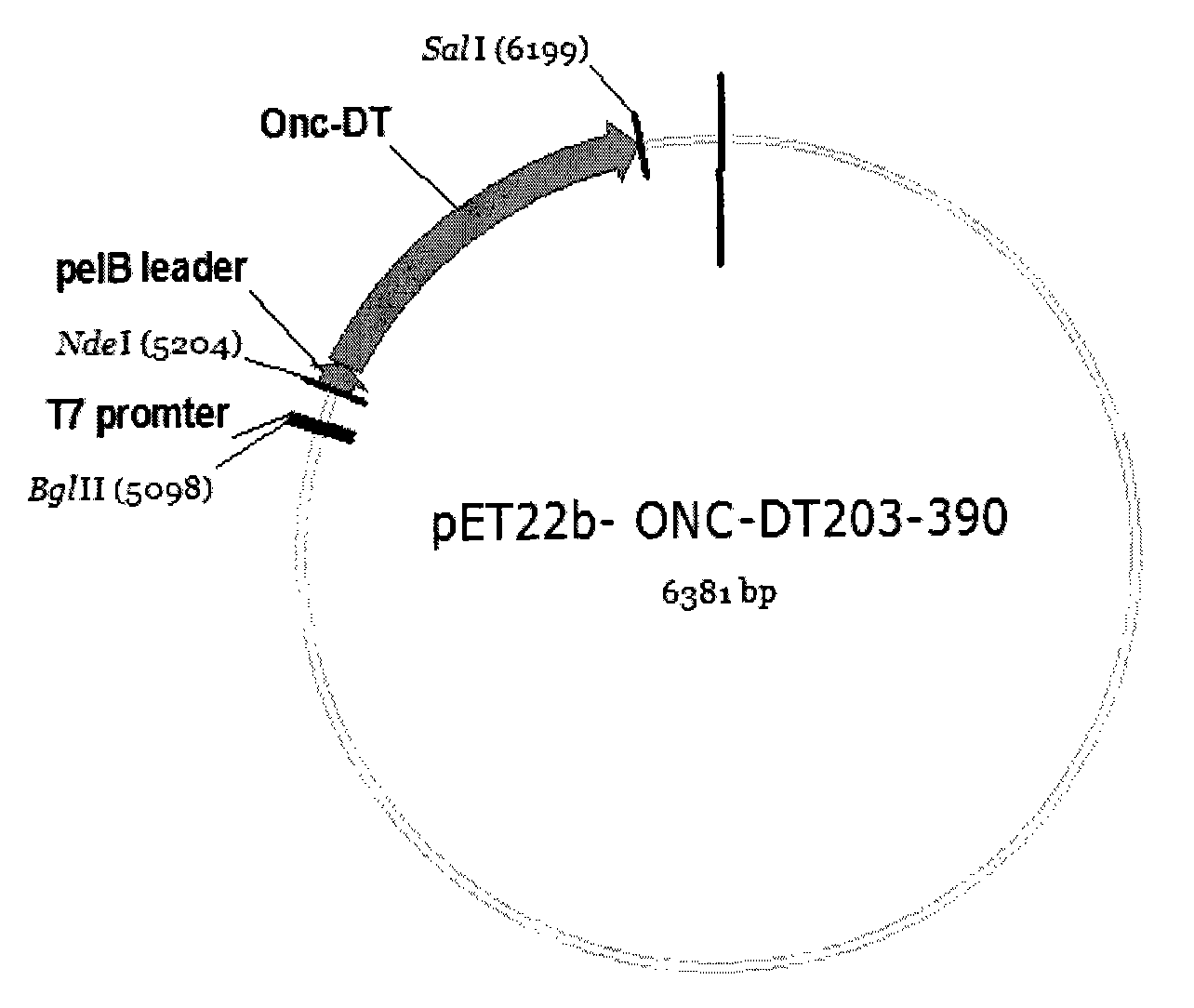
[0120] Embodiment 1Onc-DT 203-390 Synthesis of fusion gene and construction of expression plasmid in Escherichia coli
[0121] To facilitate Onc-DT 203-390 Released from the endosome to the cytoplasm, connect Onc and DT with a 16-amino acid linker peptide containing a furin restriction site. The DNA sequence of furin-DT is a whole gene synthesis. In order to facilitate PCR, Onc and furin- The DT sequences were connected, and 25 Onc terminal base sequences were added to the furin 5' end during the whole gene synthesis. See SEQ ID NO:3 for the synthetic sequence of the whole gene. The following pair of primers were designed and synthesized according to the gene sequences of Onc and DT:
[0122] Onc-DT-U (SEQ ID NO: 4):
[0123] 5'CCCAGGACTGGCTGACTTTCCA 3';
[0124] Onc-DT-L (SEQ ID NO: 5):
[0125] 5' GGGTCGACTTATTCCGGACCACCAGAAGC 3'.
[0126] Wherein, Onc-DT-U is the coding sequence of the N-terminal of Onc mature protein; Onc-DT-L is the reverse complementary sequence o...
Embodiment 2
[0128] Embodiment 2Onc-DT 203-390 Expression of fusion protein
[0129] The constructed expression plasmid was transformed into Escherichia coli BL21(DE3), and a single clone was picked overnight in 50ml LB medium and cultured overnight at 37°C. The next day, transfer 1L of TB medium at a ratio of 1:20, and culture at 37°C until A 600 =2.0, add IPTG concentration to 0.5mM to induce for 3.5 hours, collect the bacterial cells by centrifugation at 4°C, 6000rpm×10min, and the bacterial cells are used for the next step of inclusion body collection and purification.
Embodiment 3
[0130] Example 3 Inclusion Body Collection and Washing
[0131] Suspend the collected bacteria in buffer SSB_A (20mM Tris-HCl, 10mM EDTA, pH 8.0), break the bacteria with an ultrasonic breaker; centrifuge at 12000rpm×10min at 4°C to collect inclusion bodies; HCl, 1M guanidine hydrochloride, 65mM DTT, 2mM EDTA, pH 8.0) to suspend inclusion bodies, stir overnight, collect inclusion bodies at 4°C, 12000rpm×10min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com