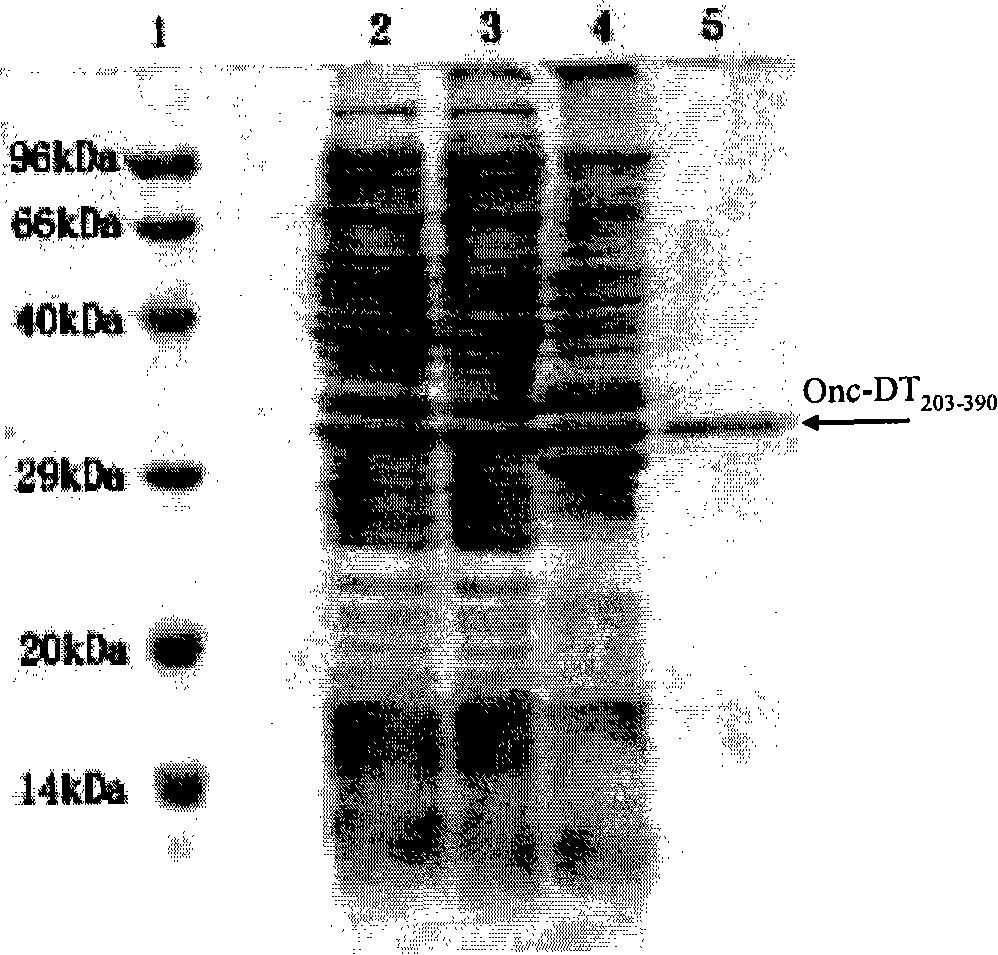Fusion protein of ribonuclease and toxin membrane translocation domain and its preparation method and use
A fusion protein and protein technology, applied in the field of DNA recombination technology and biomedicine, can solve the problems of limiting the scope of application of Onc and different sensitivities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
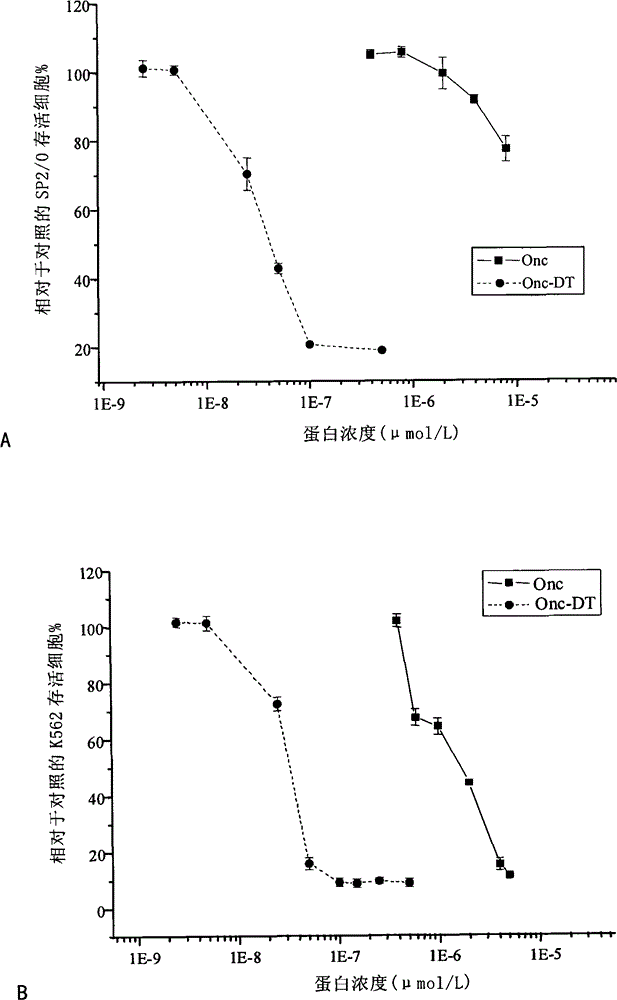
Examples
Embodiment 1
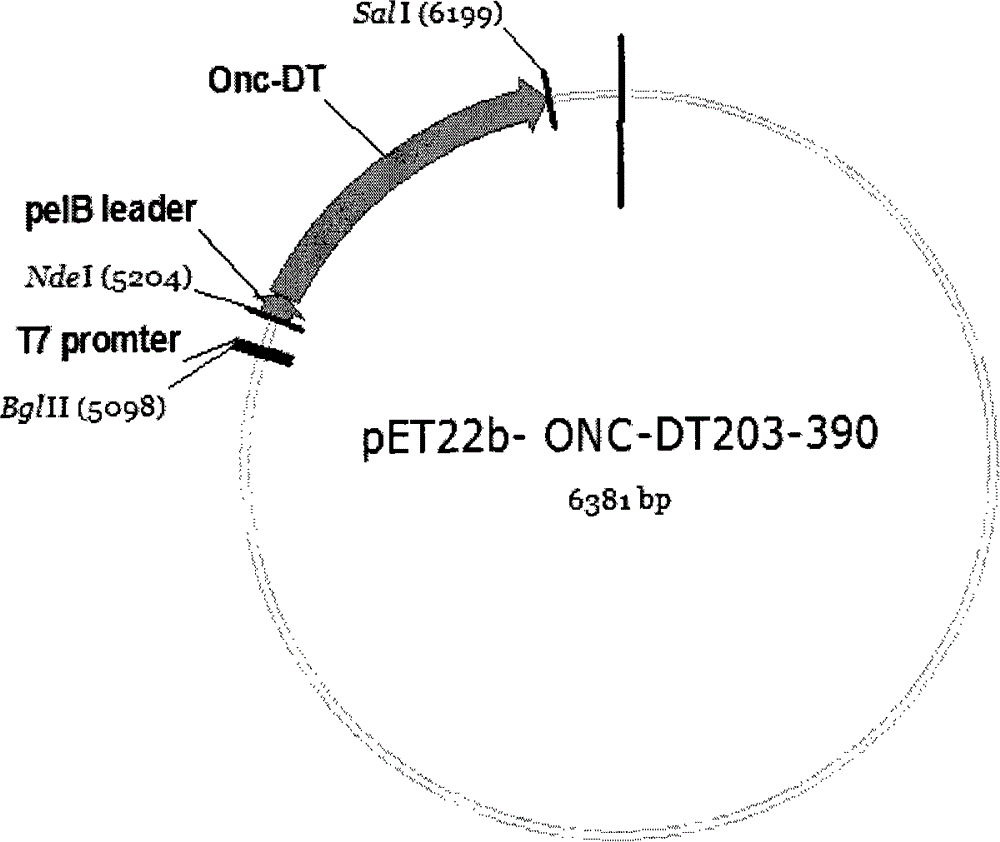
[0120] Embodiment 1Onc-DT 203-390 Synthesis of fusion gene and construction of expression plasmid in Escherichia coli
[0121] To facilitate Onc-DT 203-390 Released from the endosome to the cytoplasm, connect Onc and DT with a 16-amino acid linker peptide containing a furin restriction site. The DNA sequence of furin-DT is a whole gene synthesis. In order to facilitate PCR, Onc and furin- The DT sequences were connected, and 25 Onc terminal base sequences were added to the 5' end of furin during the whole gene synthesis. See SEQ ID NO: 3 for the synthetic sequence of the whole gene. The following pair of primers were designed and synthesized according to the gene sequences of Onc and DT:
[0122] Onc-DT-U (SEQ ID NO: 4):
[0123] 5'CCCAGGACTGGCTGACTTTCCA3';
[0124] Onc-DT-L (SEQ ID NO: 5):
[0125] 5'GGGTCGACTTATTCCGGACCACCAGAAGC3'.
[0126] Wherein, Onc-DT-U is the coding sequence of the N-terminal of the Onc mature protein; Onc-DT-L is the reverse complementary seque...
Embodiment 2
[0128] Embodiment 2Onc-DT 203-390 Expression of fusion protein
[0129] The constructed expression plasmid was transformed into Escherichia coli BL21(DE3), and a single clone was picked overnight in 50ml LB medium and cultured overnight at 37°C. The next day, transfer to 1LTB medium at a ratio of 1:20, and culture at 37°C until A 600 =2.0, add IPTG concentration to 0.5mM to induce for 3.5 hours, collect the bacterial cells by centrifugation at 4°C, 6000rpm×10min, and the bacterial cells are used for the next step of inclusion body collection and purification.
Embodiment 3
[0130] Example 3 Inclusion Body Collection and Washing
[0131] Suspend the collected cells in buffer SSB_A (20mM Tris-HCl, 10mM EDTA, pH8.0), break the cells with an ultrasonic breaker; centrifuge at 12000rpm×10min at 4°C to collect inclusion bodies; 1 M guanidine hydrochloride, 65 mM DTT, 2 mM EDTA, pH 8.0) to suspend the inclusion bodies, stir overnight, collect the inclusion bodies at 4° C., 12000 rpm×10 min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com