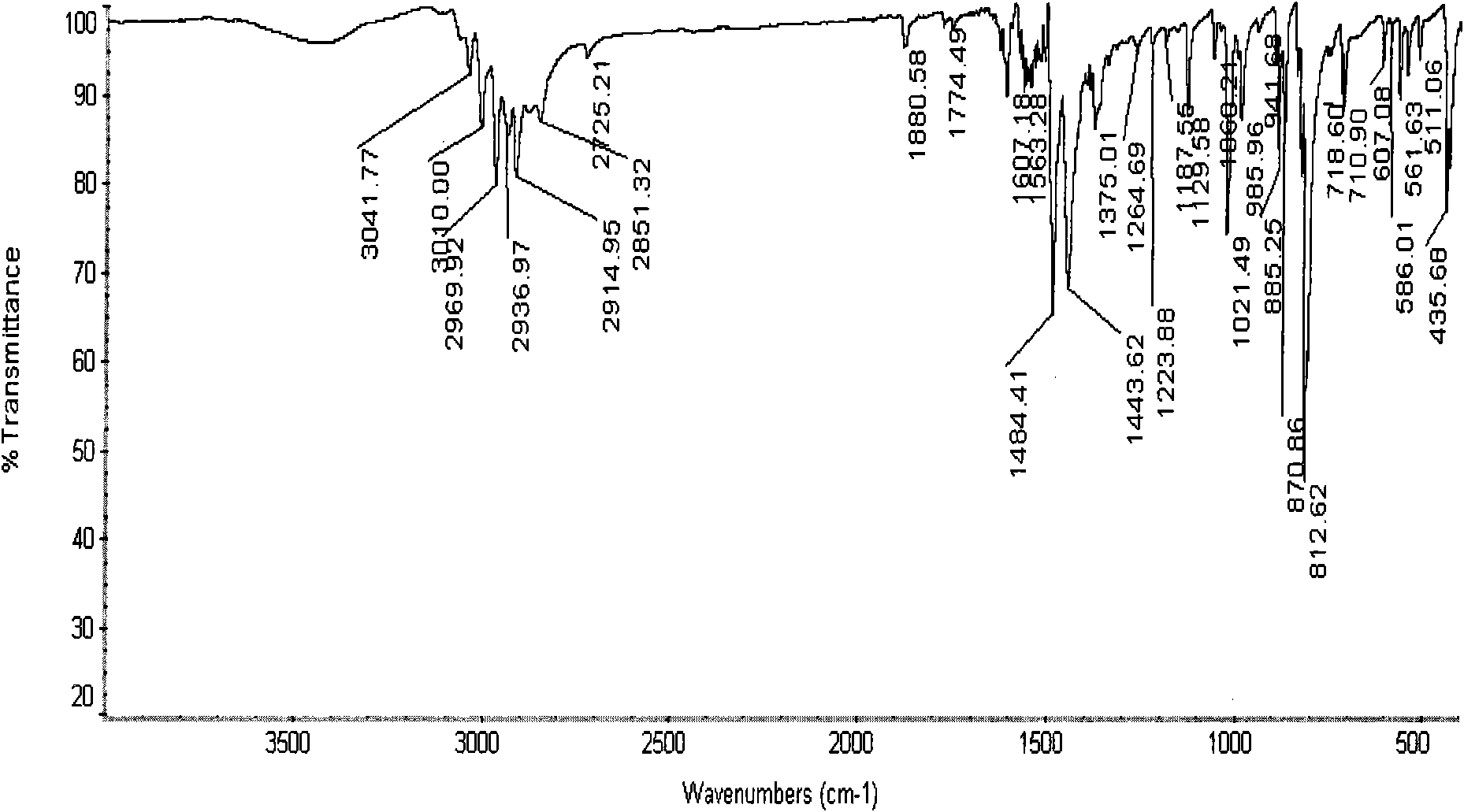
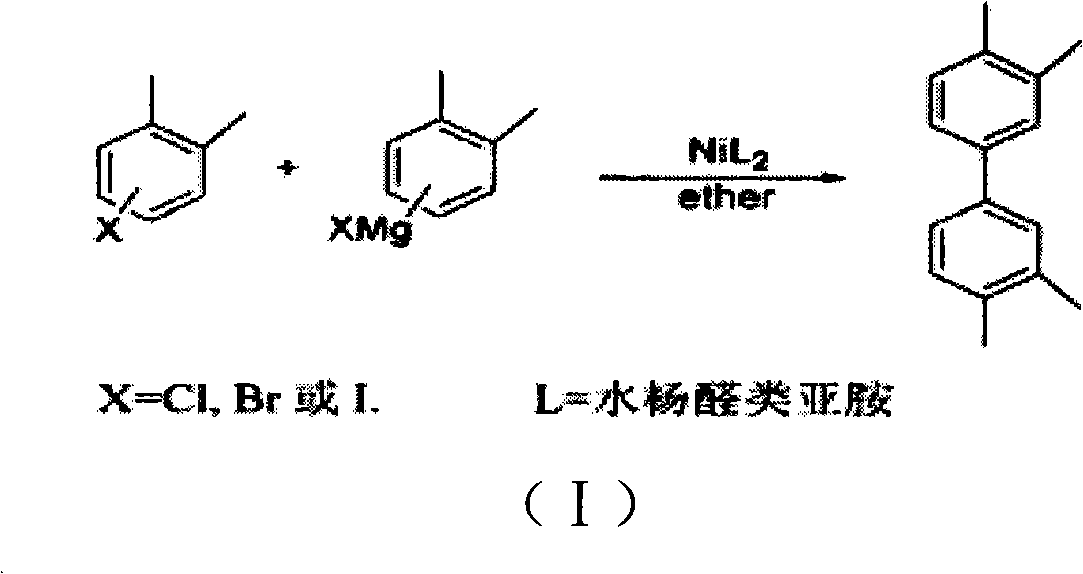
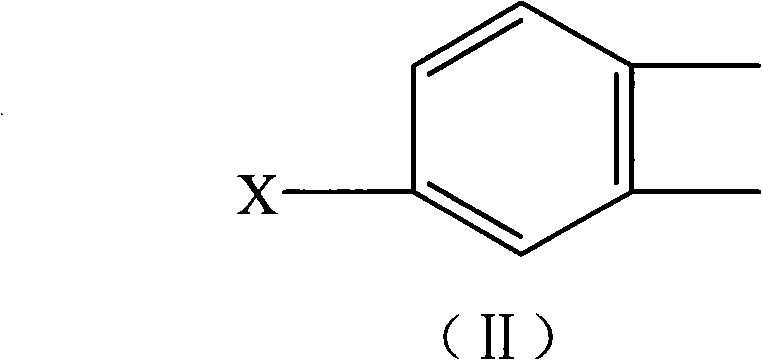
Tetramethylbiphenyl preparation method
A technology of tetramethylbiphenyl and halogenated o-xylene, which is applied in the field of preparation of biphenyl compounds, can solve problems such as difficulty in large-scale production and stringent requirements for instruments, so as to facilitate recycling, reduce equipment requirements, and simplify follow-up Effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] A preparation method of tetramethylbiphenyl, comprising the coupling reaction of halogenated aromatic hydrocarbons and the extraction of products, the reactants of the coupling reaction include using alcohol as a reducing agent, using palladium carbon as a catalyst, and using an alkaline aqueous solution as a solvent.
[0017] Wherein, the reactant halogenated arene is preferably halogenated o-xylene, which can be a compound shown in formula (II), wherein X is a halogen atom, preferably Br, Cl atom, and the halogen atom is on the benzene ring The substitution position of can be 4 or 3. The halogenated o-xylene is selected from one of 3-halogenated o-xylene or 4-halogenated o-xylene, or a mixture of the two isomers.
[0018]
[0019] Wherein, alcohol plays the role of reducing agent, can be various alcohols known to those skilled in the art, comprise monohydric alcohol; Polyhydric alcohols such as dibasic alcohol, tribasic alcohol, the present invention is preferably...
example 1
[0035] Add 120ml of distilled water, 6g of palladium carbon (70% water content, 3% palladium content in the palladium carbon), 46.67gKOH (5 / 6mol) and 30.83 g (1 / 6 mol) of bromo-o-xylene (4-bromo-o-xylene). Weigh 18.4g of glycerol (0.2mol) and dissolve it in 30ml of aqueous solution, and transfer it to a constant pressure funnel. The reaction flask was purged of air with nitrogen and sealed tightly. The temperature of the water bath was raised to 99°C, and the glycerin solution was added dropwise, and the dropping speed was controlled, and the dropping time was 1.8 hours. After the dropwise addition, keep the reaction at 99°C for 12h. Cool, filter with suction, transfer the filtrate to a separatory funnel and let stand to separate layers to obtain an organic liquid. The aqueous phase was extracted with ether and combined into the organic liquid, and the organic liquid was distilled off under reduced pressure to obtain a light yellow solid. Recrystallized with a small amount...
example 2
[0037] Add 180ml of distilled water, 7.6g of palladium carbon (70% water content, 3% palladium content in palladium carbon), 56gKOH (1mol) and 46.25 g (0.25mol) a bromo-o-xylene (containing 4-bromo-o-xylene and a small amount of 3-bromo-o-xylene). Weigh 29.4g of glycerol (0.32mol) and dissolve it in 50ml of aqueous solution, and transfer it to a constant pressure funnel. The reaction flask was purged of air with nitrogen and sealed tightly. The temperature of the oil bath was raised to 100°C, and the glycerin solution was added dropwise, and the dropping rate was controlled, and the dropping time was 1.5 hours. After the dropwise addition, the reaction was maintained at 100°C for 13h. Cool, filter with suction, transfer the filtrate to a separatory funnel and let it stand, and separate to obtain an organic liquid. The aqueous phase was extracted with ether, and combined into the organic liquid, the ether was removed by heating under reduced pressure, and the organic liquid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com