Blue-green algal virus protein N mutant, modified derivative and application thereof
A technology of cyanobacteria virus and mutants, applied in the field of cyanobacteria virus protein N mutants, can solve the problems of loss of anti-HIV activity and low activity, and achieve the effect of enhanced antiviral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
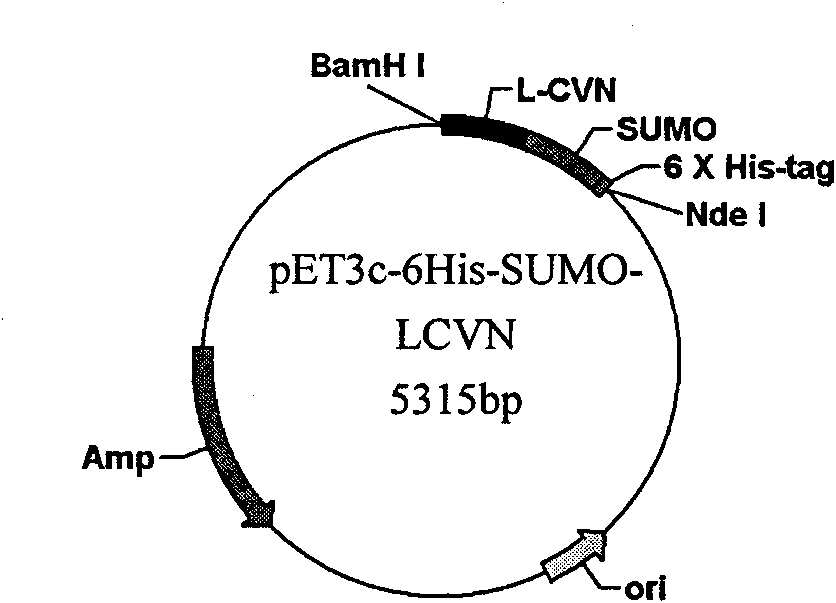
[0053] 1. Construction of recombinant plasmid pET3c-6His-SUMO-LCVN
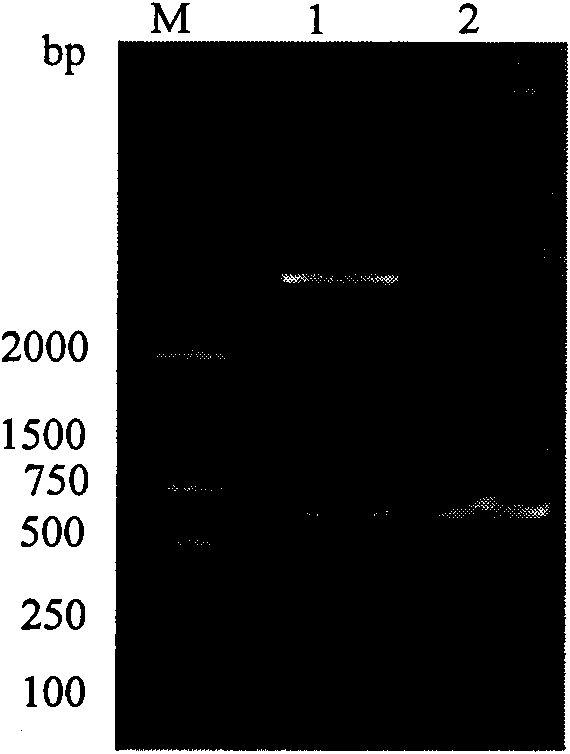
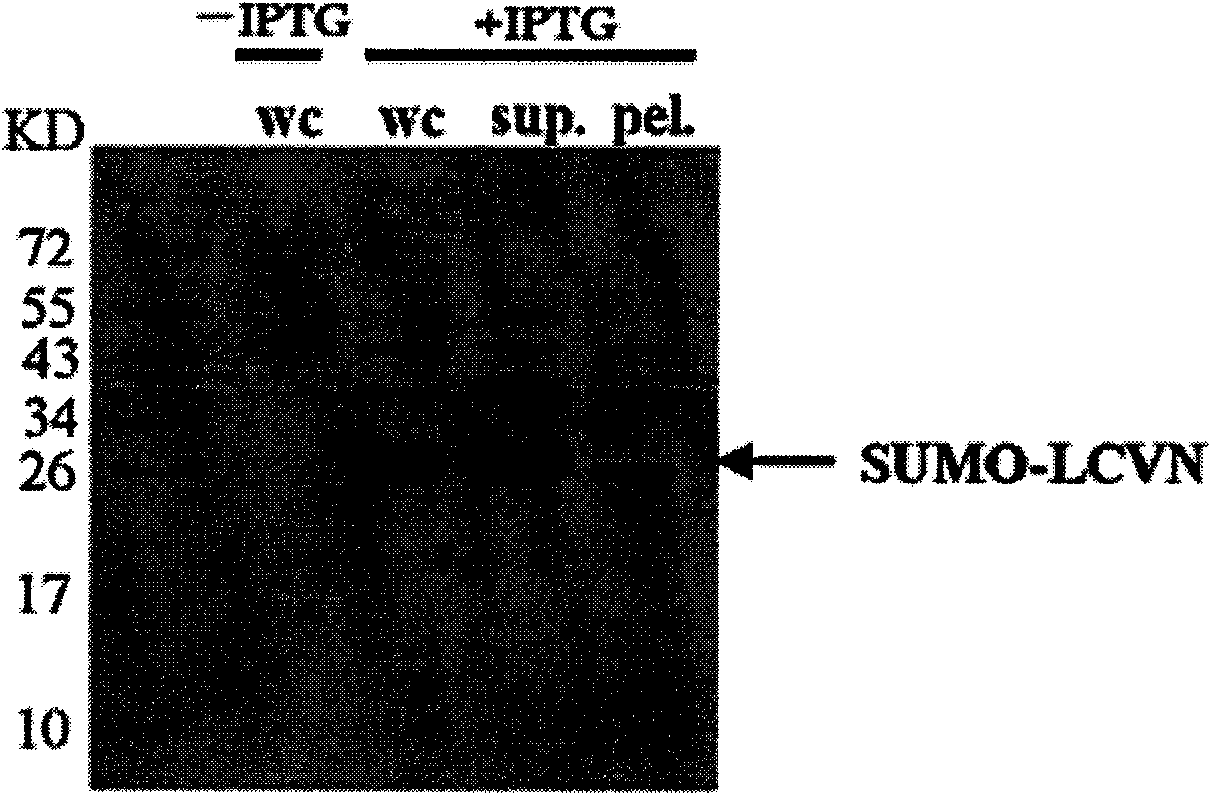
[0054] The construction and synthesis of the SUMO-L-CVN gene is carried out in two steps. First, the L-CVN gene is synthesized by two PCRs. The first PCR uses the pET3c-SUMO-CVN plasmid as a template, and F1-CVN and R-CVN as upstream and downstream primers. The reaction system was 1 ng template, 1 μM upstream and downstream primers, 20 μl Taq PCR MasterMix, added water to 40 μl, the reaction mixture was denatured at 94 °C for 1 min, annealed to 55 °C, maintained for 1 min, extended at 72 °C for 1 min, and performed 29 cycles. The reaction product was subjected to 1% agarose gel electrophoresis, and the target fragment was recovered from the gel, which was used as a template for the next round of PCR. In the second PCR, the PCR product of the previous round was used as a template, and F2-CVN and R-CVN were used as upstream and downstream primer pairs (the F2-CVN primer contained a flexible polypeptide encodin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com