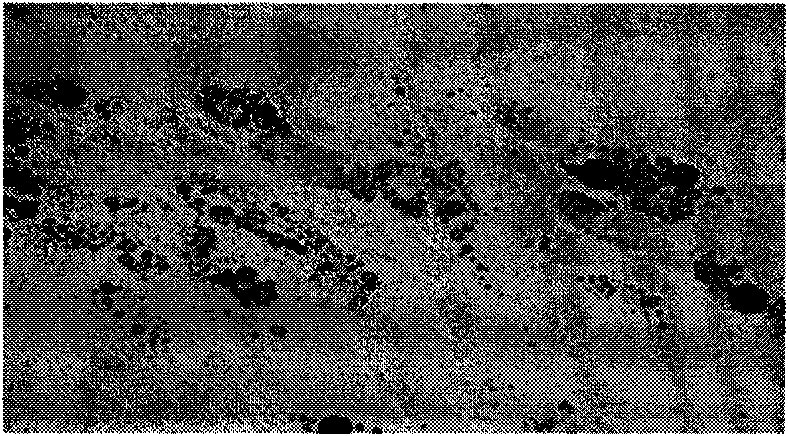Simplified method for isolation and culture of umbilical mesenchymal stem cells and application thereof in treatment of rheumatoid arthritis
A technology of mesenchymal stem cells and umbilical cords, applied in the simplified isolation and culture of umbilical cord mesenchymal stem cells and its application in the treatment of rheumatoid arthritis, which can solve the problems of large side effects, joint deformities, and high prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
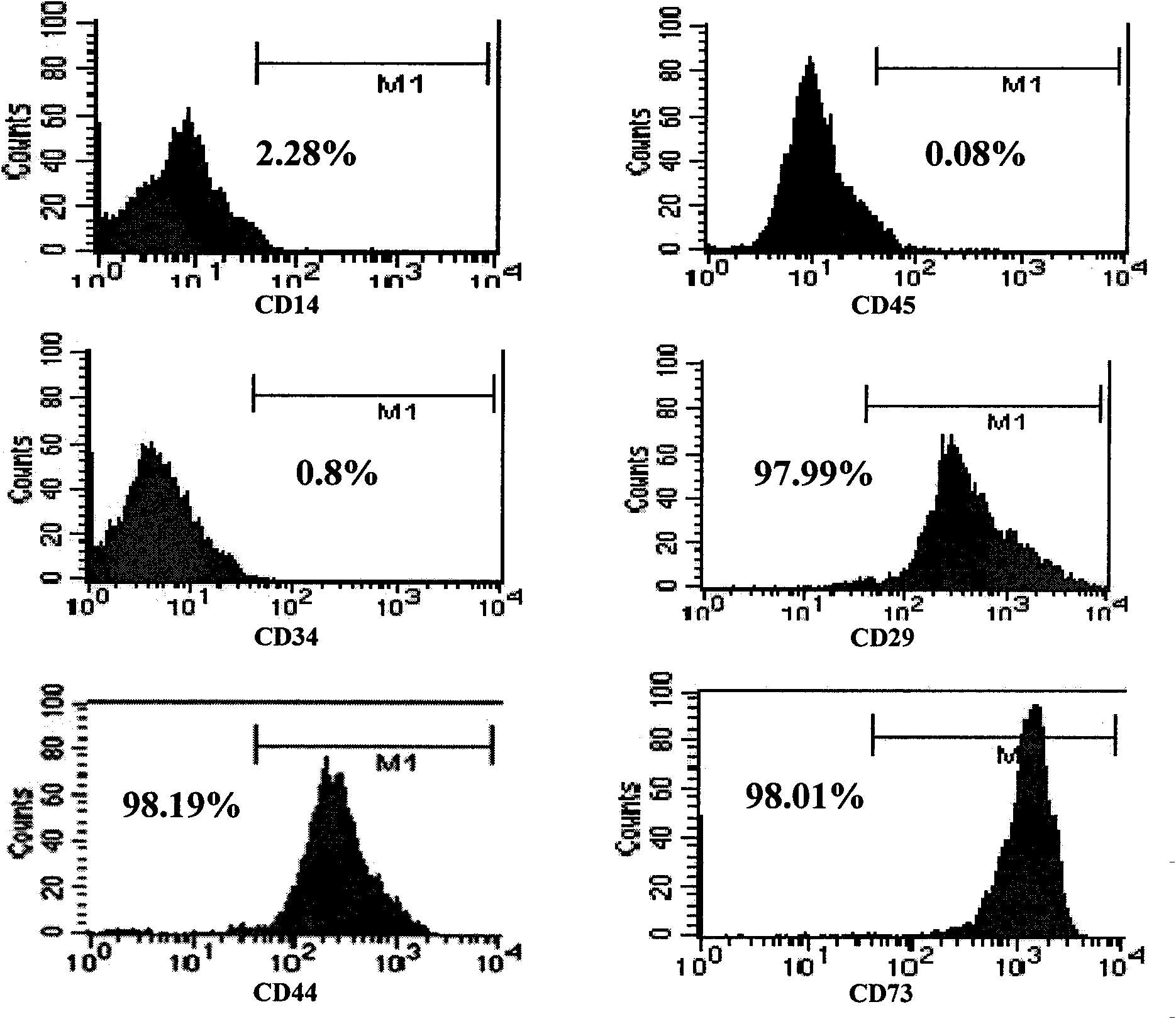
[0014] Example 1: Construction of a simplified method for isolating and culturing umbilical cord mesenchymal stem cells.
[0015] a. Take the umbilical cord from normal vaginal delivery or caesarean section, and after strict testing procedures (ABO / Rh blood type testing, HLA typing testing, syphilis antibody testing, HIV immune testing, CMV antibody testing, hepatitis B antigen antibody testing) to determine safety, no Rinse with bacterial saline to remove visible dirt and blood.
[0016] b. Aseptically cut the umbilical cord to a size of 3-5mm by hand.
[0017] c. Transfer the tissue pieces obtained in step b into a 50ml centrifuge tube, add 40ml of PBS containing 1% penicillin and streptomycin, centrifuge at 250g for 5 minutes at room temperature, and discard the supernatant.
[0018] d. The tissue block obtained in step c was cultured with 10% αMEM, and the medium was changed every 3 days.
[0019] e. At about 7 days, spindle-shaped and polygonal cells crawled out around ...
Embodiment 2
[0025] Example 2: Effect of UCMSCs on RA-FLSs proliferation ability
[0026] The synovial tissues of RA patients after knee replacement were collected, FLSs were isolated and cultured, and the cells after 3 passages were used for experiments. Divided into two groups, one group is UCMSCs and RA-FLSs co-culture group, one group is simple RA-FLSs control group, both groups are stimulated with TNF-α (10ng / ml). 120 hours, 3 H incorporation method was used to detect the proliferation of FLSs. The results showed that the proliferation of FLSs in the co-culture group was significantly inhibited in a dose-dependent manner. Even in the 5-day culture system, the addition of UCMSCs on the third day could also inhibit the proliferation of FLSs. See Figure 2.
Embodiment 3
[0027] Example 3: The effect of UCMSCs on the invasion ability of RA-FLSs
[0028] Using the 24-well invasion detection kit, UCMSCs were directly contacted with RA-FLSs for co-culture, Transwell system co-culture or the cell culture supernatant of UCMSCs was co-cultured with FLSs. After 48 hours, the number of cells passing through the membrane of the Transwell chamber was detected. After staining, the OD560 value was measured with a microplate reader. The results showed that whether UCMSCs were co-cultured with FLSs in contact, isolated and co-cultured with a transwell system, or co-cultured with the cell culture supernatant of UCMSCs and FLSs, the invasion ability of FLSs could be significantly reduced. See Figure 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com