Multifunctional nuclear shell structure drug carrier material and preparation method thereof
A technology of core-shell structure and carrier material, which is applied in the direction of pharmaceutical formulations, preparations for in vivo tests, medical preparations of non-active ingredients, etc., can solve the problems of limiting the application of rare earth luminescent materials, achieve good crystal form, and avoid components The effect of volatilization and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The following examples describe the present invention in more detail:
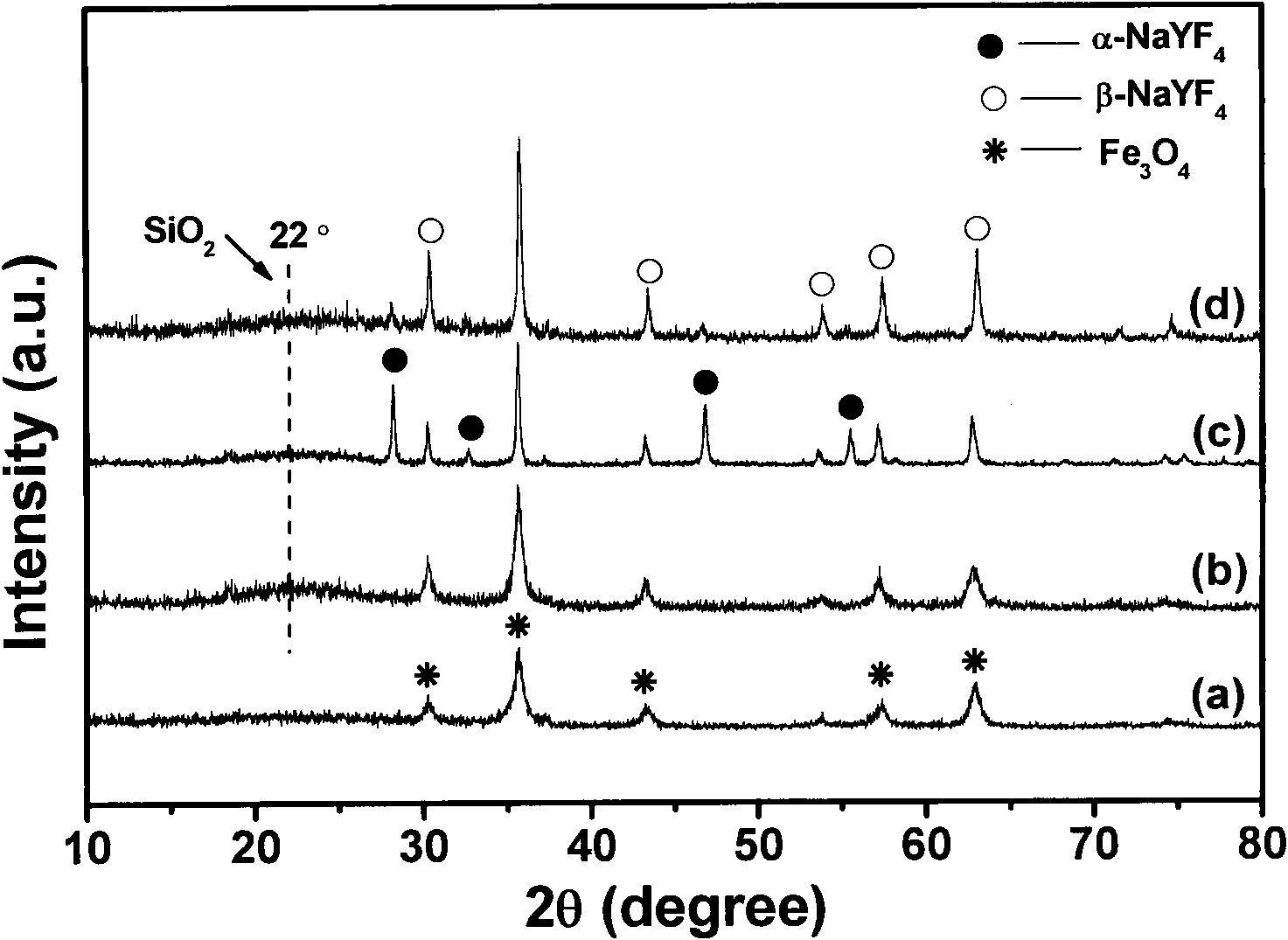
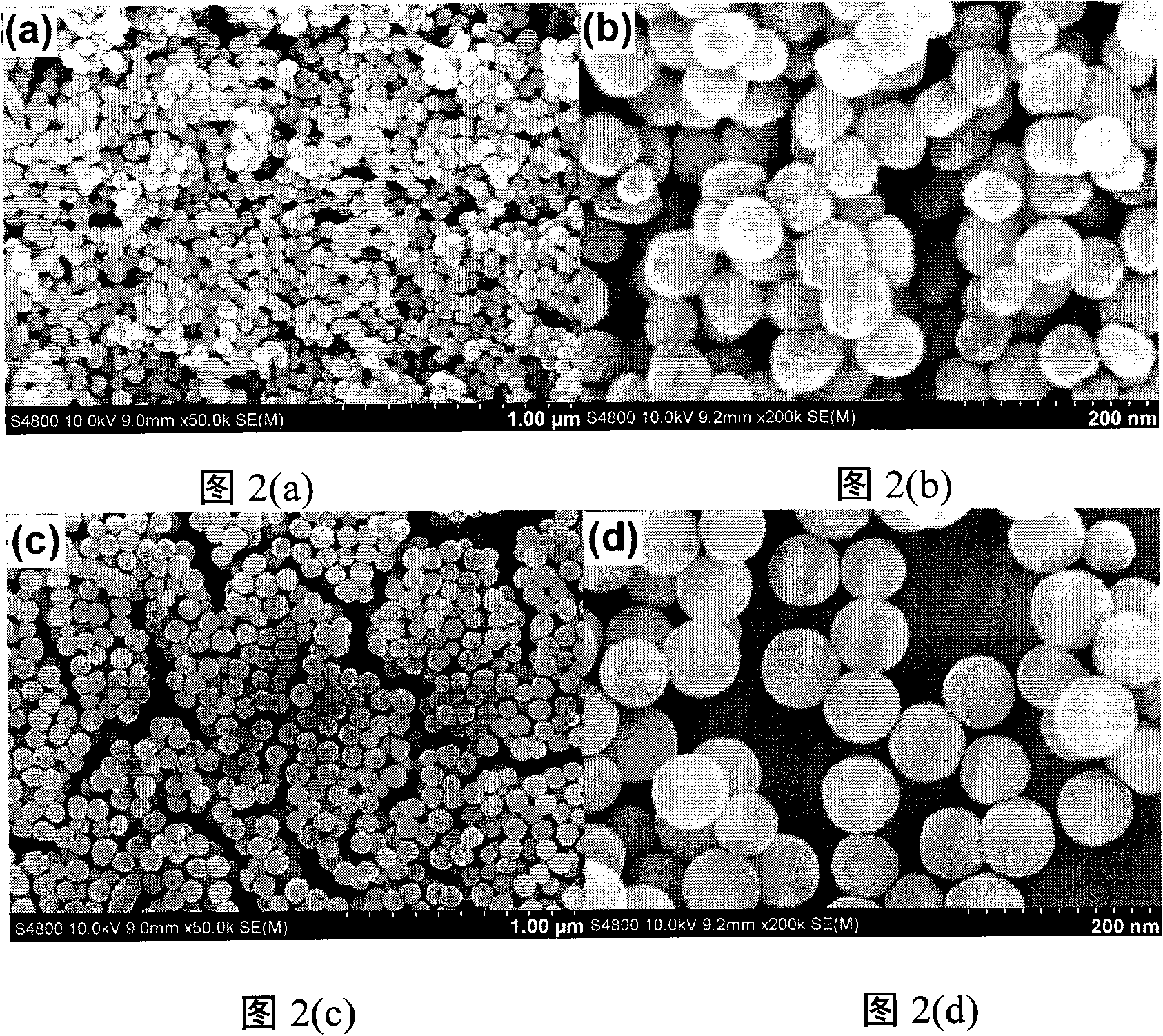
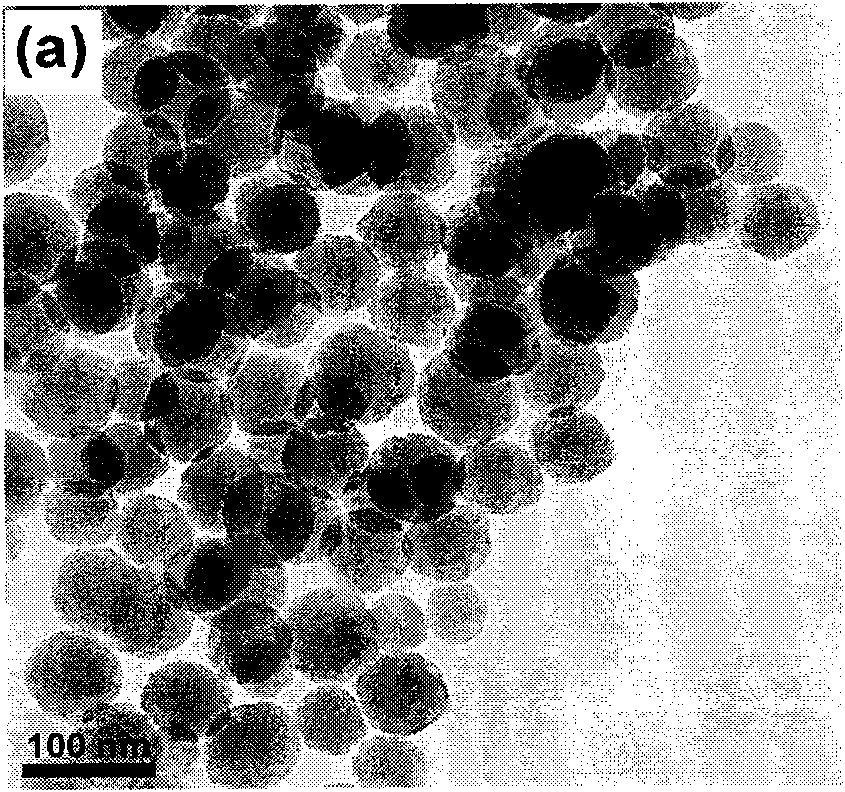
[0024] Implementation Process 1: Fe 3 o 4 @nSiO 2 @mSiO 2 @NaYF 4 : Synthesis of Yb, Er Microspheres
[0025] ① Fe 3 o 4 Preparation of nanoparticles
[0026] Using solvothermal method, firstly 1g FeCl 3 ·6H 2 O, 20mL ethylene glycol, 3g anhydrous sodium acetate and 10mL ethylenediamine were mixed and vigorously stirred for 30min to form a transparent solution. Then the obtained solution was transferred to a closed polytetrafluoroethylene-lined stainless steel reactor, and reacted at 200° C. for 12 h. After the reaction, it was naturally cooled to room temperature. The reaction product was washed several times with water and absolute ethanol, and finally dried at 80 °C for 12 h and ground into powder to obtain Fe 3 o 4 Nanoparticles.
[0027] ② Fe 3 o 4 @nSiO 2 Preparation of magnetic microspheres
[0028] core-shell Fe 3 o 4 @nSiO 2 Modified Prepared by sol-gel method. A typ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com