Lead-acid battery formation method
A technology of lead-acid battery and formation method, which is applied in the field of battery production and lead-acid battery formation, which can solve the problems of increasing capital investment in cooling equipment and cycle treatment equipment, affecting battery life, and unsatisfactory cooling effect, etc., to achieve initial capacity High, fall-off prevention, low-temperature start-up time prolongation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1: The raw electrode plates that pass the test are put into the battery tank and sealed according to the process requirements, and are assembled into a semi-finished battery; 3 The dilute sulfuric acid electrolyte was cooled in the cold acid machine for 10 hours, cooled to 20°C, and then the cooled dilute sulfuric acid electrolyte was poured into the 6-QW-54a (E) battery, and the amount of acid poured was 600ml. The deviation is ±10ml. After filling the battery with acid, put the battery into the water bath. After filling the water bath, immediately put it into the cooling circulating water for cooling in the water bath. The battery will be charged and formed within 1.5 hours of acid filling.
Embodiment 2
[0014] Embodiment 2: The qualified green plate is put into the battery tank according to the technical requirements and sealed, and is assembled into a semi-finished battery; the prepared battery has a density of 1.300g / cm2 at 25°C. 3 The dilute sulfuric acid electrolyte was circulated and cooled in the cold acid machine for 24 hours, cooled to 5°C, and then the cooled dilute sulfuric acid electrolyte was poured into the 6-QW-54a (E) battery, and the acid volume was 600ml. The deviation is ±10ml. After filling the battery with acid, put the battery into the water bath. After filling the water bath, immediately put it into the cooling circulating water for cooling in the water bath. The battery will be charged and formed within 1.5 hours of acid filling.
Embodiment 3
[0015] Embodiment 3: The green electrode plates that pass the test are put into the battery tank and sealed according to the process requirements, and are packed into semi-finished batteries; 3 The dilute sulfuric acid electrolyte was circulated and cooled in the cold acid machine for 18 hours, cooled to 15°C, and then the cooled dilute sulfuric acid electrolyte was poured into the 6-QW-54a (E) battery, and the amount of acid poured was 600ml. The deviation is ±10ml. After filling the battery with acid, put the battery into the water bath. After filling the water bath, immediately put it into the cooling circulating water for cooling in the water bath. The battery will be charged and formed within 1.5 hours of acid filling.
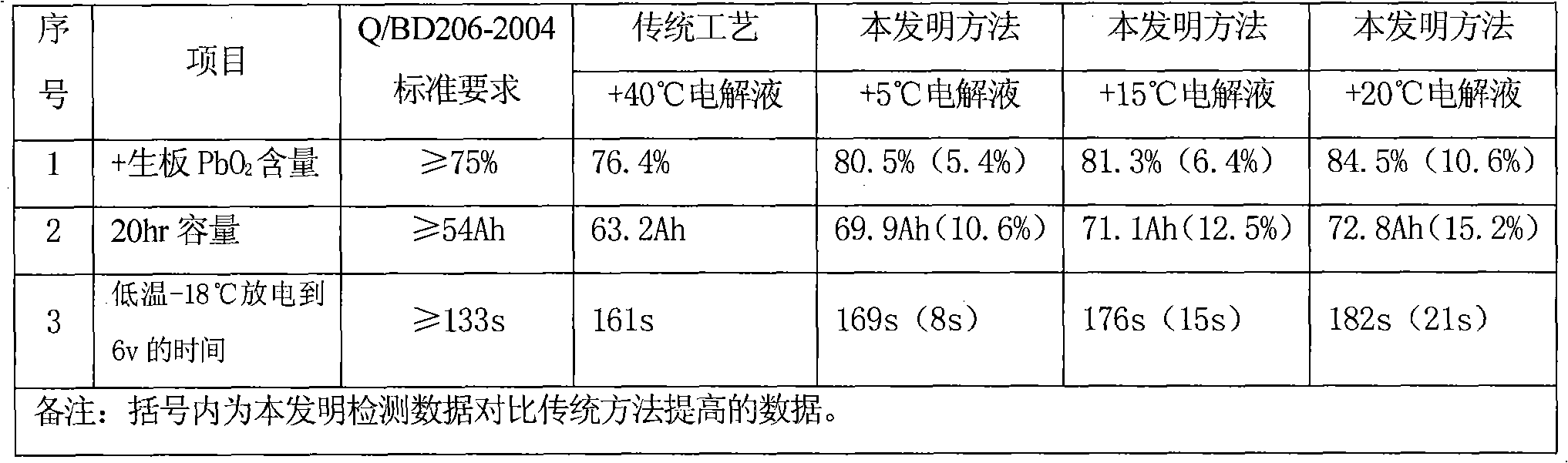
[0016] The performance test of the above embodiment is shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com