Prodrugs and methods of making and using the same
A kind of technology of prodrug and compound, applied in the field of prodrug and its preparation and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
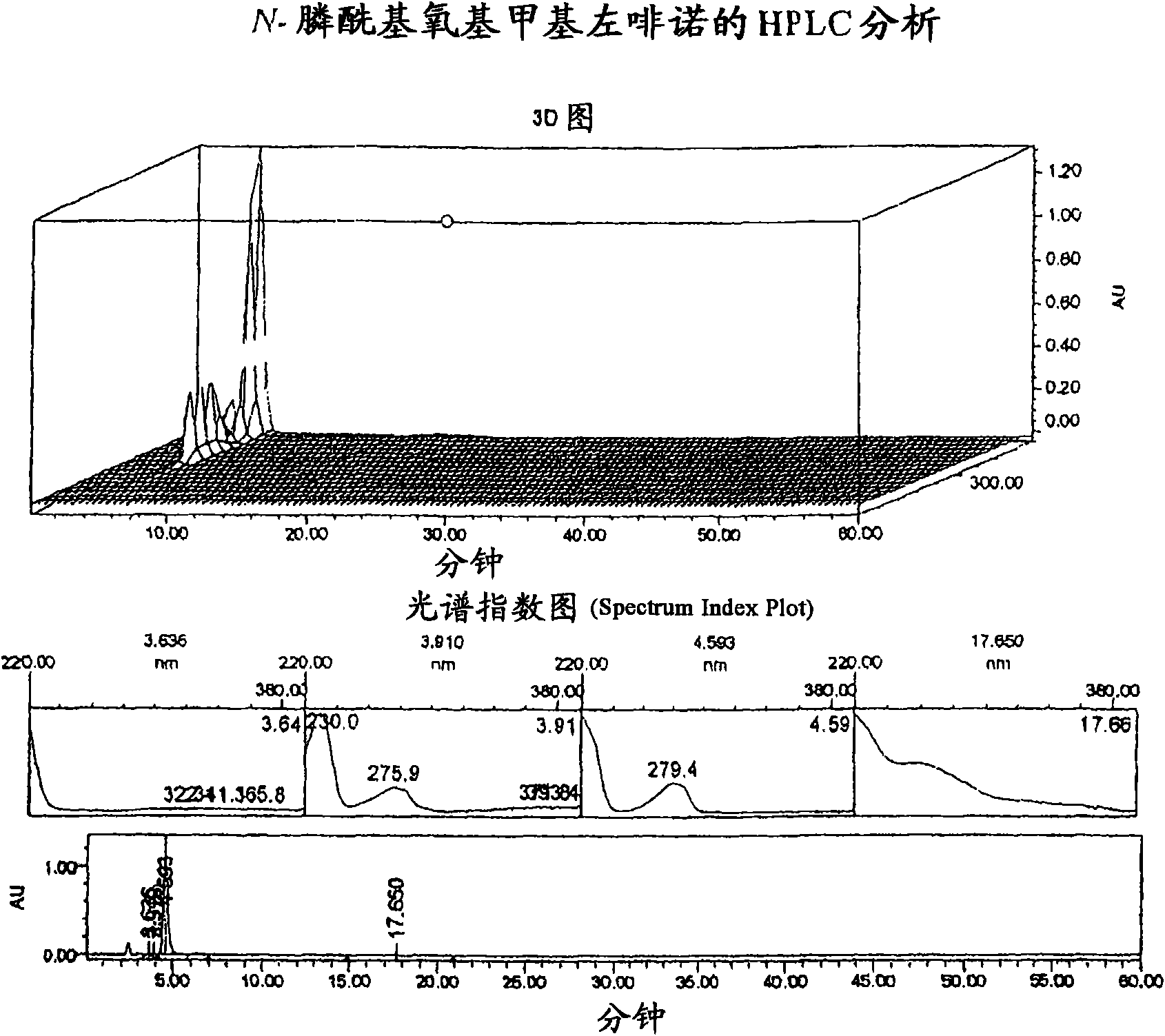
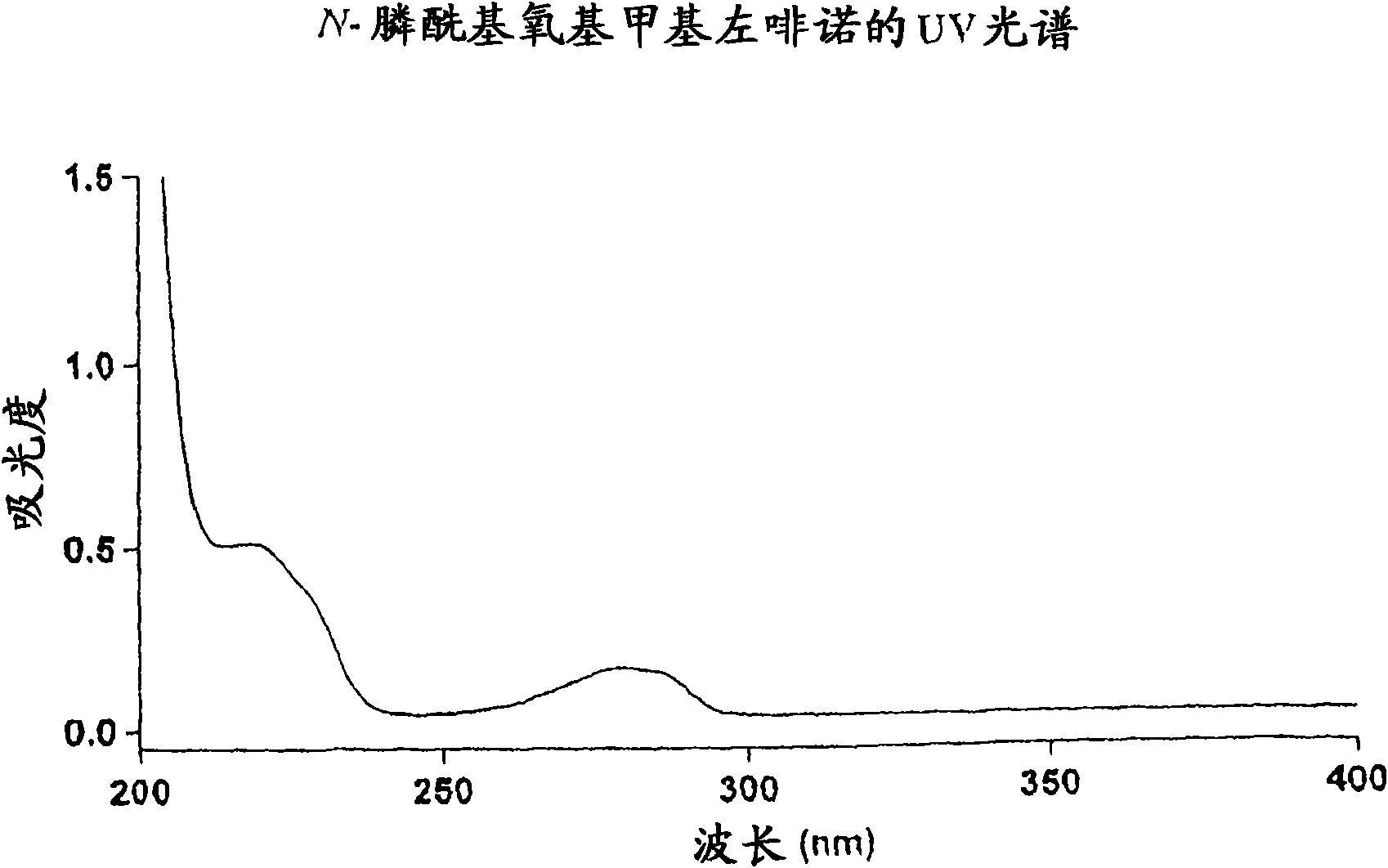
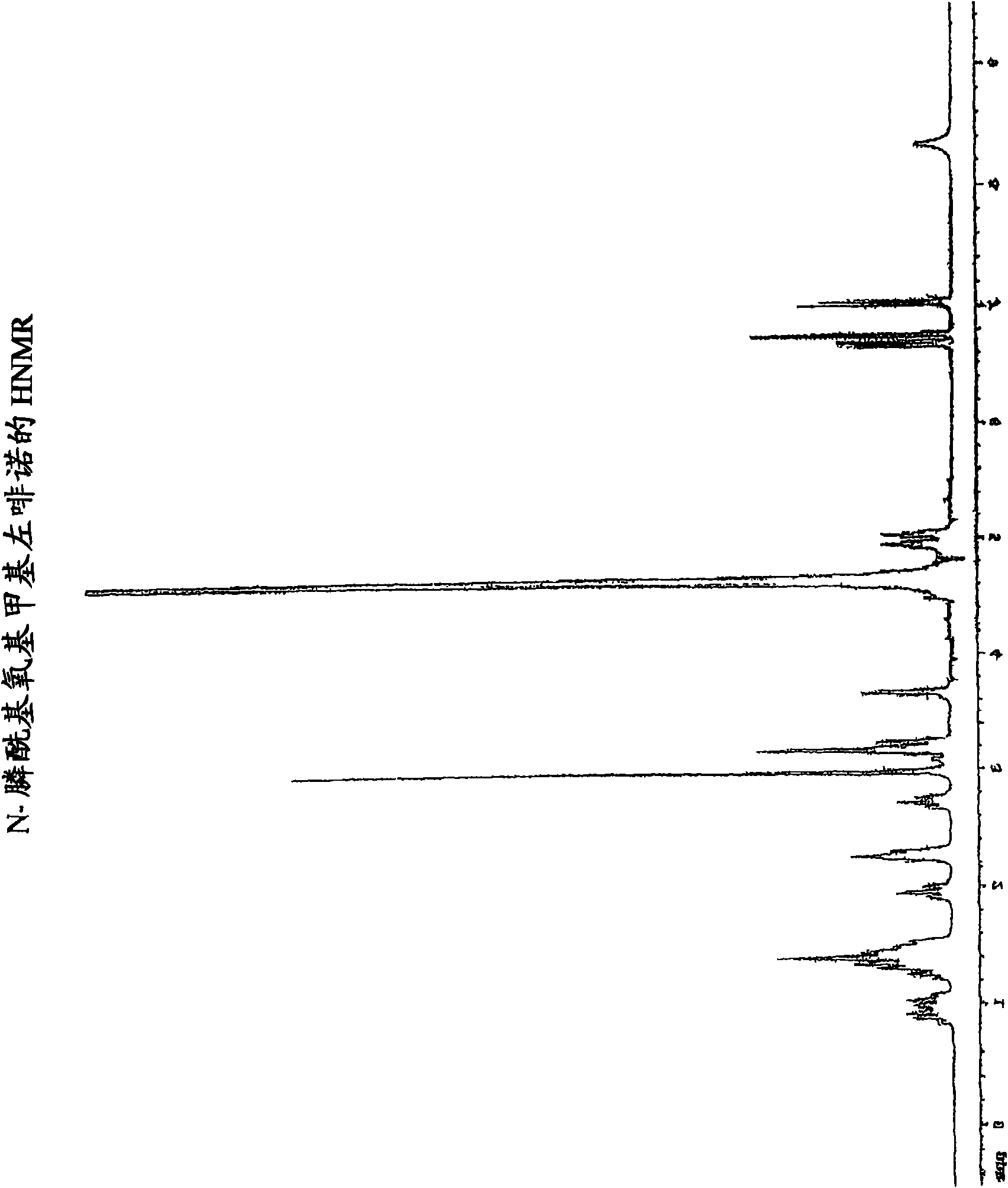
[0227] Embodiment 1: the synthesis of N-phosphonooxymethyl levorphanol
[0228] Preparation of di-tert-butyl chloromethyl phosphate (3). Di-tert-butyl chloromethyl phosphate was prepared according to Scheme 1 of the reaction example.
[0229]
[0230] Example reaction scheme 1
[0231] Di-tert-butyl phosphate (2). To a solution of di-tert-butyl phosphite (1) (25 g, 128 mmol) and potassium bicarbonate (7.g, 77.9 mmol) in 28 mL of water stirred in an ice bath was added 6 equal parts of permanganese over a period of 1 hour Potassium phosphate powder (34.7 g, 220 mmol). The purple mixture was stirred for an additional 45 minutes at room temperature. Thelerite (5 g) was added and the resulting mixture was stirred at 60°C. The mixture was filtered through a celite filter cake and the filter cake was washed with 3x50 mL of water. The combined filtrates were mixed with 10 g of sorite and stirred at 60°C for 30 minutes. The mixture was filtered again through celite, and the...
Embodiment 2
[0239] Example 2: Chemical Hydrolysis Studies
[0240]The aqueous solution stability of N-phosphonooxymethyl levorphanol at 37°C, pH 1.2, 6 and 8 was determined by comparing sample solutions at the specified time points indicated in the data table below. Various pH (1.2, 6 and 8) PBS solution. The solubility of N-phosphonooxymethyl levorphanol at pH 8 in PBS was found to be approximately 137 mg / mL. Levorphanol (free base) dissolves less than 1 mg / mL by sonication at 37°C. A 50 [mu]L sample was collected at the t=0 time point. The remaining solution was then divided into 5 aliquots (175 μL each) using microcentrifuge tubes labeled according to the sampling time point. The airtight, sealed vials were then incubated at 37°C in a temperature-controlled water bath. At each time point, the appropriate vial was removed from the water bath and a 50 μL sample was withdrawn for HPLC analysis. Table 1 below presents the HPLC analytical data indicating the test conditions, samplin...
Embodiment 3
[0247] Example 3: Enzymatic Hydrolysis Studies
[0248] Determination of N-phosphonooxymethyllevorphanol by HPLC by comparing the area under the peak of the sample solution at specific time points (0, 0.5, 1.25, 3, 5.5, and 23 hours) with the area under the peak of a freshly prepared sample Stability at pH 8, 37°C in the presence of alkaline phosphatase (EC 3.1.3.1; Sigma-Aldrich catalog #P7923). Alkaline phosphatase (400 units) was added to 10 mL of alkaline phosphatase stabilization buffer (APSB; Sigma-Aldrich catalog #A4955) maintained at 37°C. The pH of the solution was determined by dropping 25 μL of the solution onto a calibrated color-pH indicator strip (pH 2-9, Darmstadt, Germany) and comparing the color to the calibrated range. N-phosphonooxymethyllevorphanol (3 mg) was added to 3 mL of the above enzyme+buffer and incubated at 37°C. At each sampling time point, 200 μL aliquots were injected into labeled microcentrifuge tubes, each containing 50 μL of Alkaline Phos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com