Therapeutic agent for ulcerative colitis comprising mizoribine
A technology of ulcerative colitis and mizoribine, applied in the field of ulcerative colitis therapeutic medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
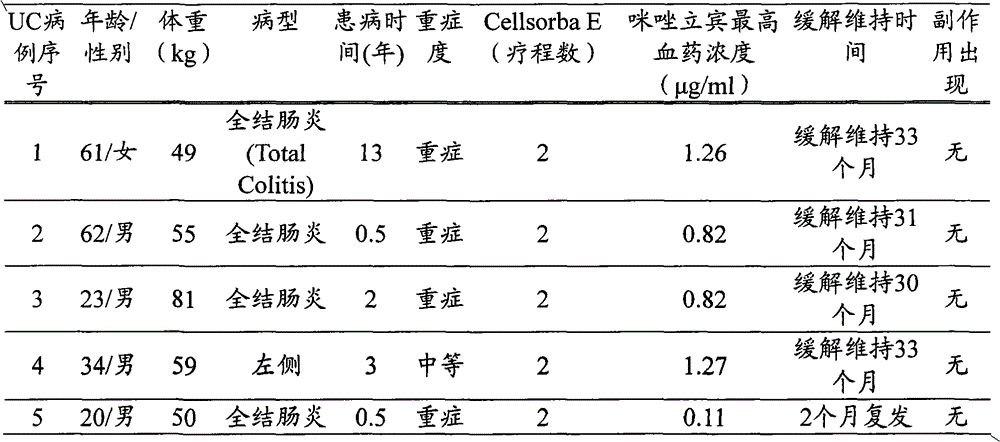
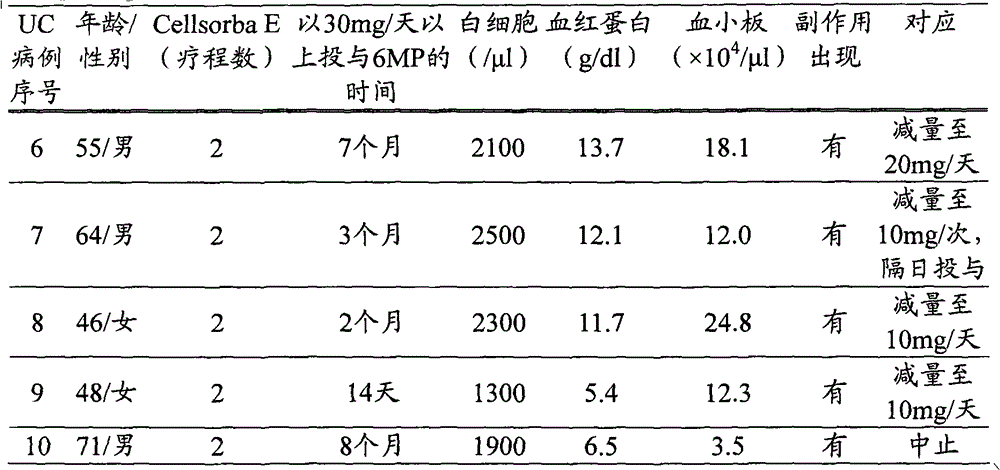
[0057] (1) Target patients
[0058] Four patients with ulcerative colitis (UC, ulcerative colitis) at the hospital to which the present inventor belongs (Oita Red Cross Hospital, hereinafter the same) were studied. Case numbers 1 to 4 were patients with a history of side effects caused by oral administration of 6-MP or azathioprine for ulcerative colitis of moderate severity or higher in the severity classification of the Ministry of Health, Labor and Welfare. Hereinafter, the ulcerative colitis patients targeted in Comparative Example 1 and Comparative Example 2 also had the same drug history.
[0059] (2) The determination method of the highest plasma concentration of mizoribine
[0060] The determination of the maximum plasma concentration of mizoribine was carried out according to the usual method (JChromatogr. 1988 Nov 18; 432: 340-5). After oral administration of mizoribine to the patient, 2 to 4 hours after the highest blood concentration can be obtained, collect 2ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com