A therapeutic or prophylactic agent for corneal epithelial lesions and/or conjunctival epithelial lesions
A purpose, recovery agent technology, applied in the field of therapeutic or preventive agents for corneal epithelial lesions and/or conjunctival epithelial lesions, can solve the problems of easy damage, dry eyes, difficulty in using contact lenses, etc., and achieves a long consumption history. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] production of n-3 fatty acids deficient animals
[0057] Feeds for experimental animals generally contain n-3 fatty acids with an appropriate DHA content; therefore, in order to generate n-3 fatty acid deficient animals, special feeds that do not contain n-3 fatty acids must be used. In addition, normally raised animals receive sufficient DHA intake from the placenta and mother's milk from before birth to weaning, so it is not easy to produce target n-3 fatty acid deficient animals within only one generation. With these facts in mind, an n-3 fatty acid deficient diet containing 7% fats and oils was prepared using AIN93G (the standard refined diet composition for mouse and rat nutrition studies published by the American Institute of Nutrition) as the basal diet. Feed (n-3Def, linolenic acid: 14.4%; alpha-linolenic acid: 0.1%). N-3 fatty acids (n-3Adq, linolenic acid: 14.3%; α-linolenic acid: 2.6%) containing α-linolenic acid (18:3, n-3) were used as normal feed. Fema...
Embodiment 2
[0079] Using the same fish oil as in Example 1 (refined fish oil EPA-28 (containing more than 28% EPA and more than 12% DHA) produced by Nippon Suisan Co., Ltd.), the effect on human corneal epithelial lesions was confirmed.
[0080] Protocol Summary
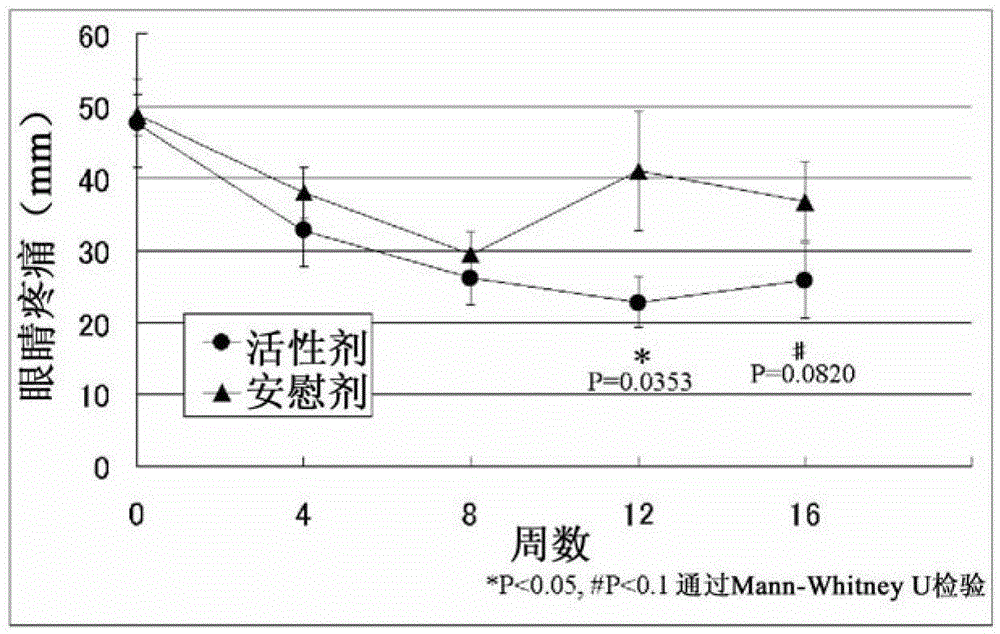
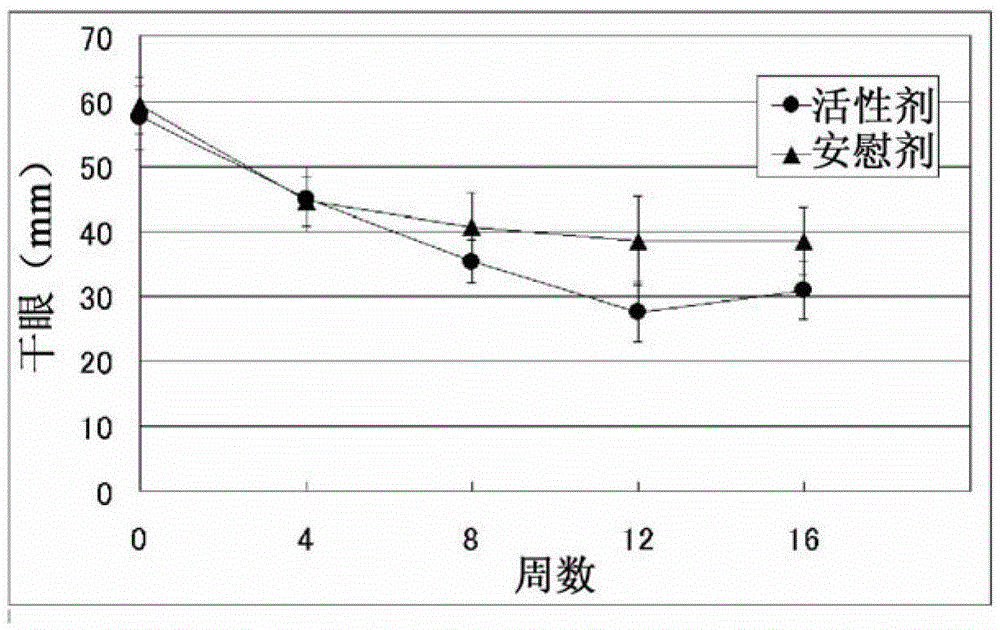
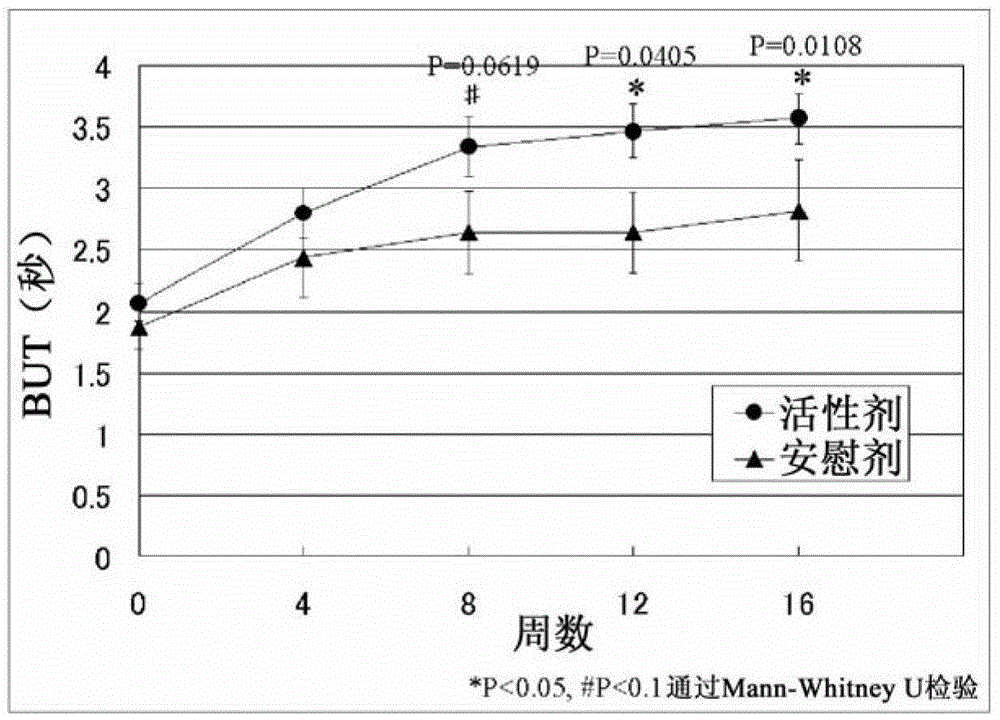
[0081] A double-blind trial was used to evaluate the efficacy of EPA and DHA on 27 subjects who were diagnosed with dry eye disease according to the diagnostic criteria for dry eye disease (2006, Dry Eye Society). Take active or placebo supplements for 12 weeks while continuing normal dry eye treatment with eye drops etc. The active group administered refined fish oil capsules (15 capsules) for a daily intake of 1,245 mg of EPA and 540 mg of DHA. The placebo group was administered medium-chain triglyceride (MCT) capsules (15 capsules). Efficacy was determined at a total of 5 times: before administration; weeks 4, 8 and 12 after administration; and week 4 of the follow-up period after administration.
[0082] Evaluate the fo...
Embodiment 3
[0094] Taking patients with symptoms of eye fatigue as objects, a double-blind randomized controlled trial was used to compare the effect between the active group and the placebo group, wherein the active group took 162mg of EPA, 784mg of DHA, and 59mg of anthocyanidin in the form of capsules daily and 17 mg of lutein, and the placebo group took capsules containing medium-chain triglycerides.
[0095] result
[0096] The number of subjects was 11 in the active group and 9 in the placebo group. Subjective symptom assessment was performed 4 weeks after the start of administration, at which time there was a statistically significant improvement in dryness in eye symptoms in the active group compared to the placebo group. In addition, in the active group, significant improvements were observed in eye symptoms in terms of fatigue, blinking, eye hyperemia, and blurred vision 4 weeks after the start of administration compared with before administration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com