Compound and application thereof in preparation of anti-tumor pharmaceutical, food or cosmetic
An anti-tumor effect and compound technology, applied in the preparation of organic compounds, anti-tumor drugs, applications, etc., can solve the problems that have not been solved, the route of administration is limited, and achieve excellent anti-tumor effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0193] Embodiment 1. The making of tablet
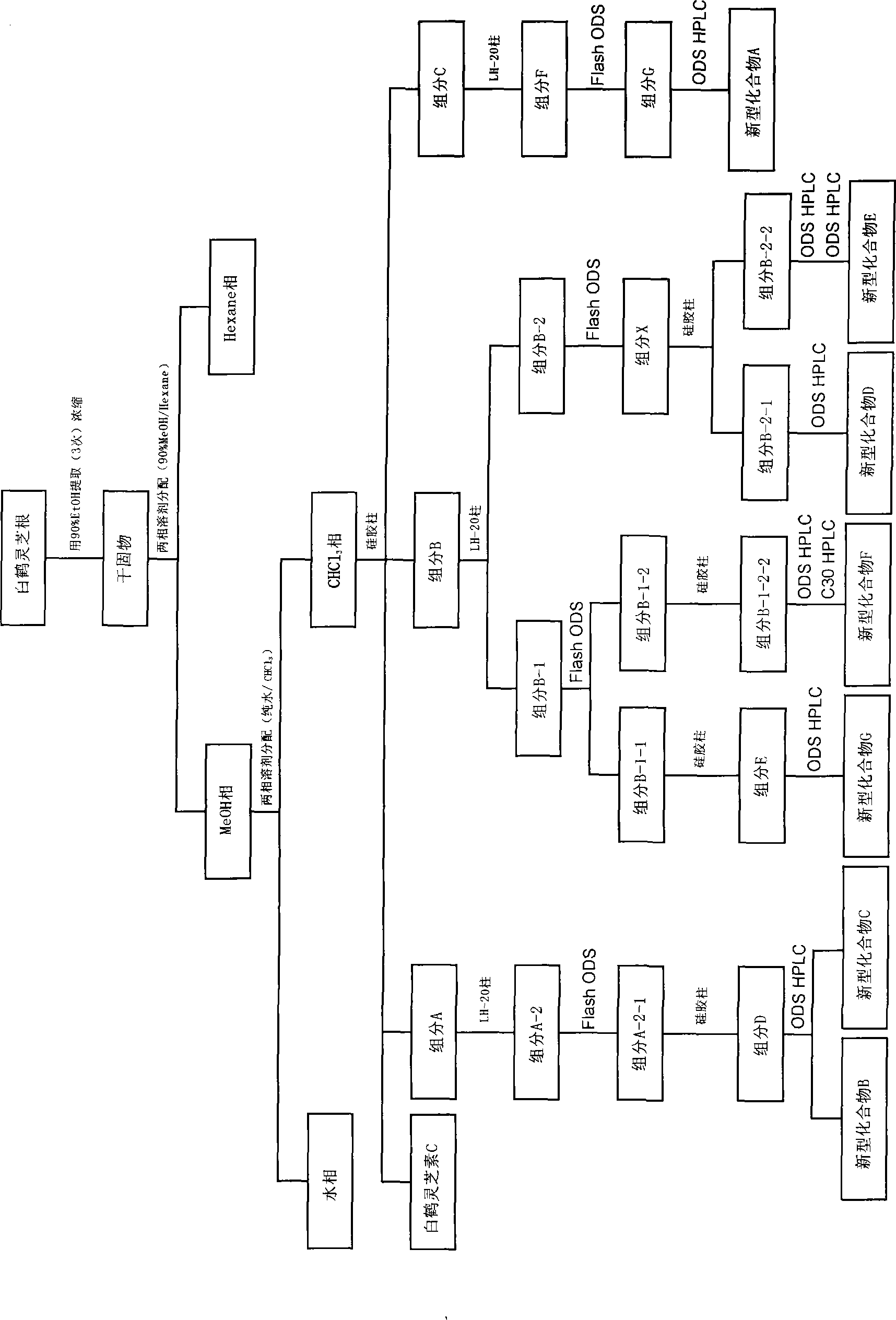
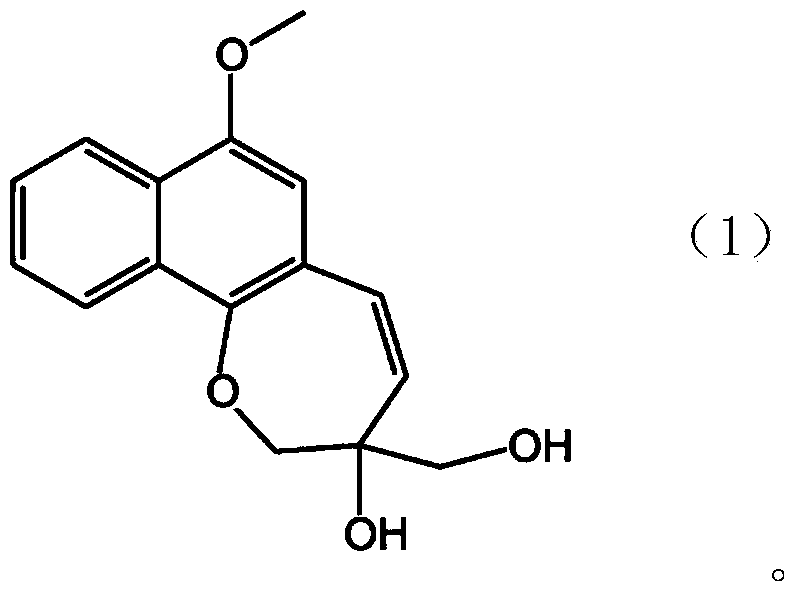
[0194] Tablets (500 mg per tablet) were prepared using the novel compound A that was classified and isolated according to the method described in the above "1-1. Novel compound A (1) Classification and isolation of the novel compound A" with the following prescription.
[0195]
[0196] New compound A (0.2g), dry cornstarch (2g), talc (1.8g), and calcium stearate (0.2g) were added and mixed to lactose (95.8g). Next, using a single-punch tablet press, tablets were prepared according to a conventional method.
Embodiment 2
[0197] Embodiment 2. the making of hard capsule
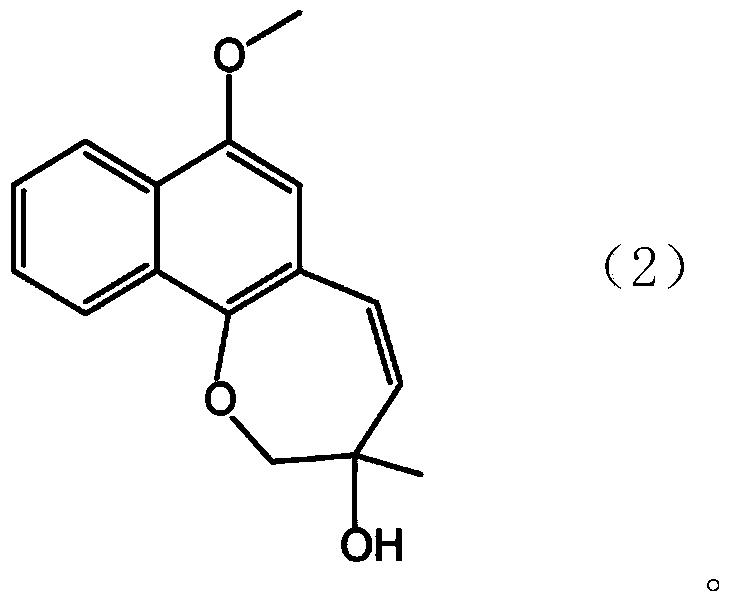
[0198] Using the novel compound B that was classified and separated according to the method described in the above "1-2. Novel compound B, C (1) Classification and separation of the new compound B and C", hard capsules were produced with the following prescription (average per 1 capsule 360mg).
[0199]
[0200] Lactose (220 g) and cornstarch (110 g) were added and mixed to novel compound B (5 g), and an aqueous solution of hydroxypropylcellulose (25 g) was added thereto, followed by kneading. Next, pellets were prepared by a conventional method using an extrusion pelletizer. Hard capsules are prepared by filling the granules into gelatin hard capsules.
Embodiment 3
[0201] Embodiment 3. the making of soft capsule
[0202] Using the novel compound C that was classified and separated according to the method described in the above "1-2. Novel compound B, C (1) Classification and separation of novel compound B and C", soft capsules were prepared with the following prescription (average per 1 capsules 170mg).
[0203] Novel compound C 0.5mg
[0204] Soybean Oil 169.5mg
[0205] (preparation method)
[0206] The novel compound C (0.5 g) was added and mixed to soybean oil (169.5 g). Soft capsules were then produced by filling into soft capsules according to the usual method using a rotary-mode automatic molding machine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com