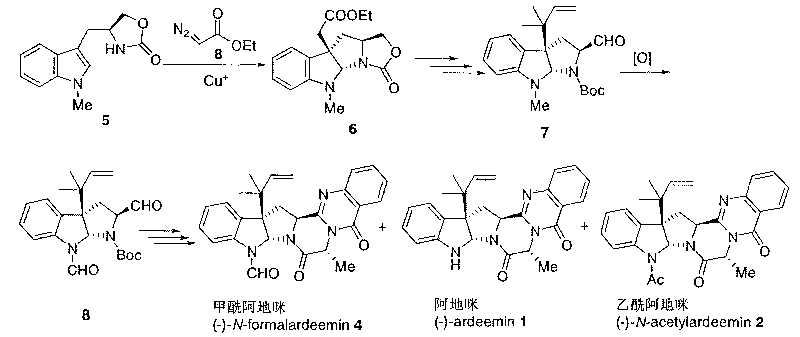
High-activity anti-caner new medicament formalardeemin for inhibiting multi-drug resistance of tumor cells
A technology of formylademide and base formylademide, which is applied in the field of highly active anti-cancer drug formylademide, which can inhibit the multidrug resistance of tumor cells, can solve the problem of low yield and separation of acetylademide Mi 2 is difficult and restricts in-depth research and development and utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
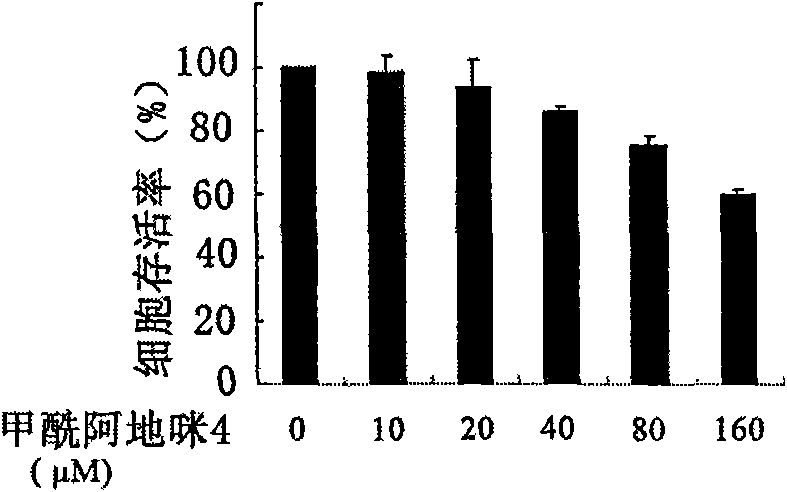
[0025] Example 1 Inhibitory effect of formylademide 4 on the proliferation of human breast cancer MCF-7 cells
[0026] experimental method:
[0027] Human breast cancer MCF-7 cells were cultured with PRMI1640 (Hyclone) medium containing 10% fetal bovine serum, and placed in a 37°C, 5% CO2 incubator. Tumor cells 0.7×10 4 Each well was inoculated on a 96-well plate, and after culturing for 24 hours, formylademide 4 with a final concentration of 10 μM, 20 μM, 40 μM, 80 μM, and 160 μM was added respectively. The control group was added with DMSO, and a zero-adjusted group (only culture medium was added) ), with three replicate wells for each group. The 96-well plate was placed in a 37°C, 5% CO2 incubator for another 72 hours, and the MTT colorimetric method was used to detect the survival rate of tumor cells, that is, 10 μl of 5 mg / ml MTT (Sigma, USA) reagent was added to each well, and the culture was continued for 4 hours. Remove the supernatant, wash with PBS, add 100 μl of ...
Embodiment 2
[0030] Example 2 Inhibitory effect of formylademide 4 on the proliferation of human cervical cancer Siha cells
[0031] experimental method:
[0032] Human cervical cancer Siha cells were cultured with PRMI1640 (Hyclone) medium containing 10% fetal bovine serum, and placed in a 37°C, 5% CO2 incubator. Tumor cells 0.7×10 4 Each well was inoculated on a 96-well plate, and after culturing for 24 hours, different concentrations of formylademide 4 were added according to Example 1. After continuing to cultivate for 72 hours, the survival rate of tumor cells was detected according to the method in Example 1.
[0033] result:
[0034] Such as Figure 4 As shown, formylademide 4 dose-dependently inhibited the growth of human cervical cancer Siha cells cultured in vitro.
Embodiment 3
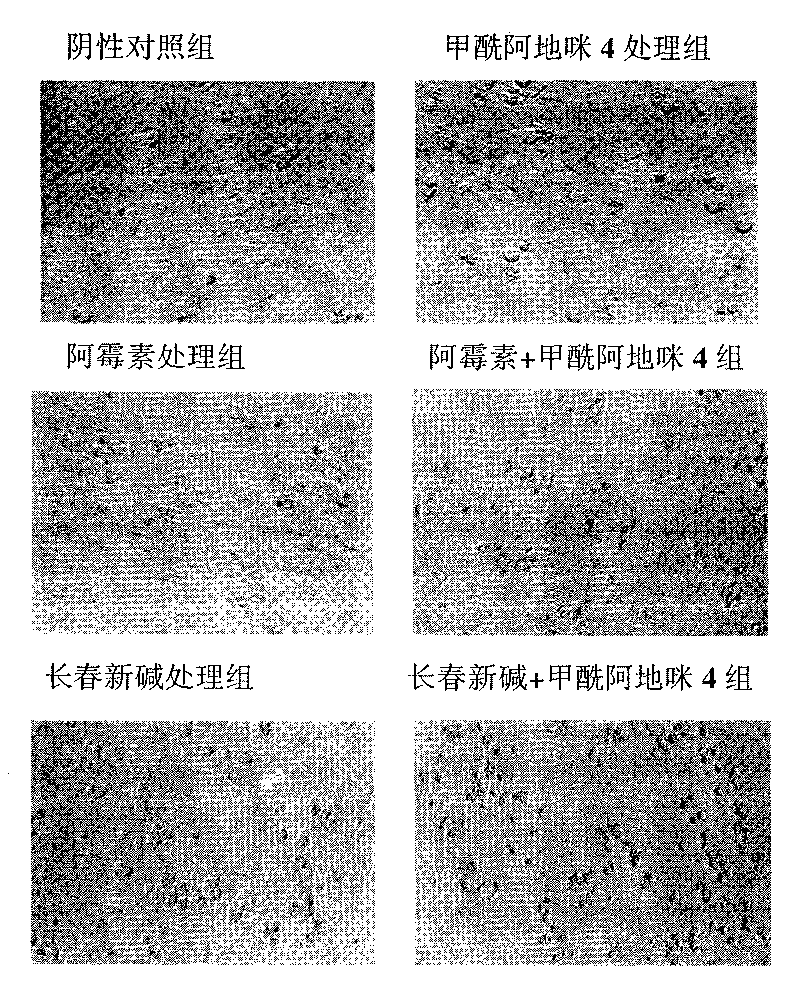
[0035] Example 3 Formylademide 4 significantly increases the sensitivity of human cervical cancer Siha cells to doxorubicin
[0036] experimental method:
[0037] The culture conditions of human cervical cancer Siha cells are the same as those in Example 2. Tumor cells 0.7×10 4 Inoculate each well on a 96-well plate, and after culturing for 24 hours, add doxorubicin (0.4 μg / ml, Italian Pharmacia) or different concentrations of formylademide 4 (20 μM, 40 μM, 80 μM, 160 μM) , or add doxorubicin and different concentrations of formylademide 4 at the same time, add DMSO to the control group, and set up a zeroing group (only add culture medium), and set three replicate wells for each group. Place the 96-well plate in a 37°C, 5% CO2 incubator for another 72 hours, and then remove the lactic acid
[0038] Hydrogenase (LDH) release assay, using lactate dehydrogenase detection kit (Cyto Tox 96 Non-Radioactive Cytotoxicity Assay, Promega, Cat. No. G1780), to detect cell death. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com