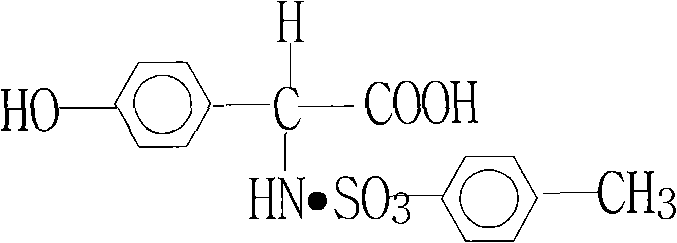
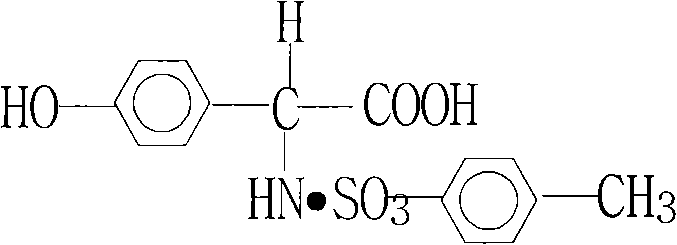Technology for hydrolyzing D-(-)-p-hydroxyphenylglycine-p-toluenesulfonate
A technology of p-hydroxyphenylglycine and toluene sulfonate is applied in the production field of pharmaceutical chemical intermediates and can solve the problems of low yield, poor quality and high cost of L-p-hydroxyphenylglycine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] 1. First add 2500 kg of pure water into the 5000L reactor, start stirring and put in 1000 kg of purified D salt, and open the steam valve to raise the temperature.
[0016] 2. After heating up and dissolving, add 10 kg of activated carbon for decolorization, keep warm at 85-90°C for 30 minutes, and then filter while it is hot.
[0017] 3. Add 5 kg of EDTA catalyst to the filtered mother liquor, neutralize it with ammonia water, and adjust the pH between 4.2-4.4.
[0018] 4. Cool down to 30°C and keep warm for 2 hours, then centrifuge.
[0019] 450-460 kg of pure L-p-hydroxyphenylglycine can be obtained, and the quality is as follows:
[0020] Content≥99.5%
[0021] Absorption≤0.050
[0022] Optical rotation: -156°~-161°
[0023] Single impurity≤1ppm
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com