Lipoprotein analysis by differential charged-particle mobility
A lipoprotein, charged technology, applied in the analysis of materials, biological material analysis, material electrochemical variables, etc., can solve the problems of wrong quantitative results, lipoprotein confusion, wrong qualitative results, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1 - Using D 2 Comparison of Purified Lipoproteins with O and Low Salt Solutions
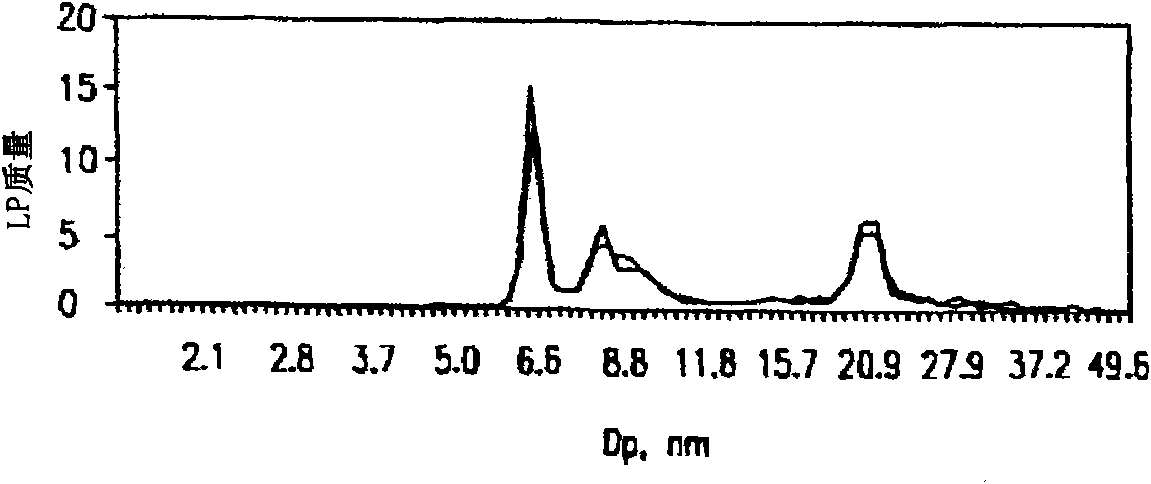
[0108] Use a low-density salt solution (1.151 g / mL) (ie, "low-density salt sample") or D 2 Serum samples (25uL) were treated with O (200uL each). Samples were centrifuged at 223,000×G for 3.7 hours (low density salt samples) or 2 hours (D 2 O). After removing the top 100 uL after centrifugation, low density salt samples were dialyzed against ammonium acetate solution and diluted to 1:200 prior to differential charged particle mobility analysis. Before differential charged particle mobility analysis, D 2 O samples were directly diluted to 1:200 with ammonium acetate after centrifugation. The results of the differential charged particle mobility analysis are in figure 2 displayed in .
Embodiment 2
[0109] Example 2 - Effect of Purification on Apo A, Apo B and TC Recovery
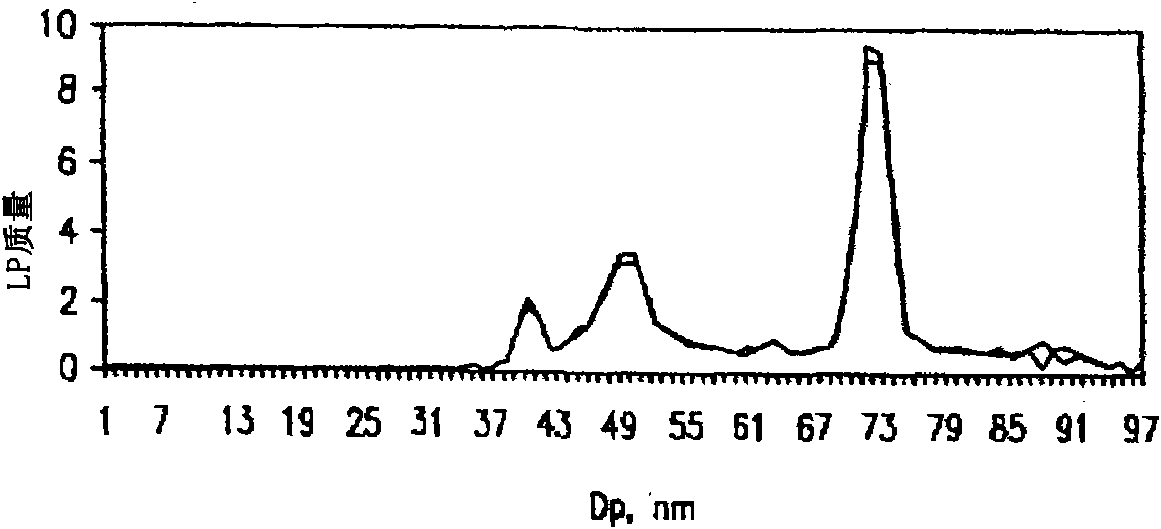
[0110] To assess HDL (Apo A1) in the use of D 2 Whether the loss is preferred in the step of O, such as image 3 The three samples shown in (i.e., 749, 1043, 14: arbitrary and unique patient identification numbers) were subjected to lipoprotein separation using D 2 O along with the RGD / DS solution (7.5 / 2.5 mg / mL, respectively) solution to remove the albumin. Each sample was prepared in six replicates. The top 100 uL of each isolation was analyzed for Apo Al (HDL), Apo B (LDL, IDL, VLDL) and total cholesterol (TC) content. Plasma or serum apolipoproteins AI and B were measured by standard ELISA using a commercially available monoclonal capture antibody (Biodesign International, Saco, MN) and an anti-human goat polyclonal detection antibody in a noncompetitive sandwich immunoassay Apolipoproteins AI and B were purified and biotinylated (International Immunology Corp., Murrieta, CA). Concentrations w...
Embodiment 3
[0111] Example 3 - Effect of different RGDs on recovery of lipoprotein fractions
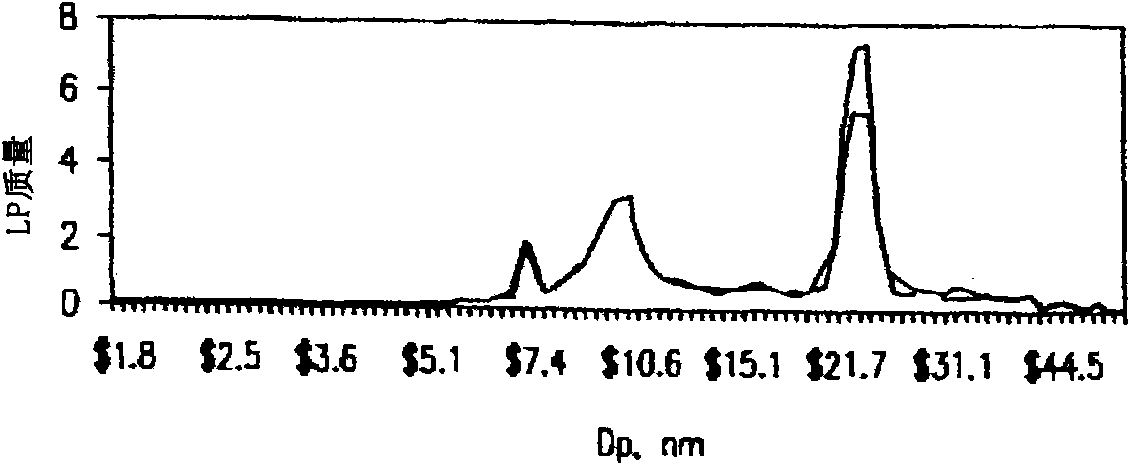
[0112] being paved in D 2 Serum samples were mixed with different amounts of RGD (10, 15, 20, 25 mg / mL) and incubated on ice for 15 minutes before topping the O cushion. After centrifugation at 223,000 x G for 120 minutes, the top 100 uL was removed and diluted 1:200 with ammonium acetate solution. The samples were then analyzed by differential charged particle mobility analysis. result in Figure 4 displayed in .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com