Insulinotropic peptide derivative wherein its n-terminal amino acid is modified
一种促胰岛素、释放肽的技术,应用在胰岛素样生长因子、激素肽、胰高血糖素等方向,能够解决未得到详细说明书的支持、没有提到类似物活性和性质等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
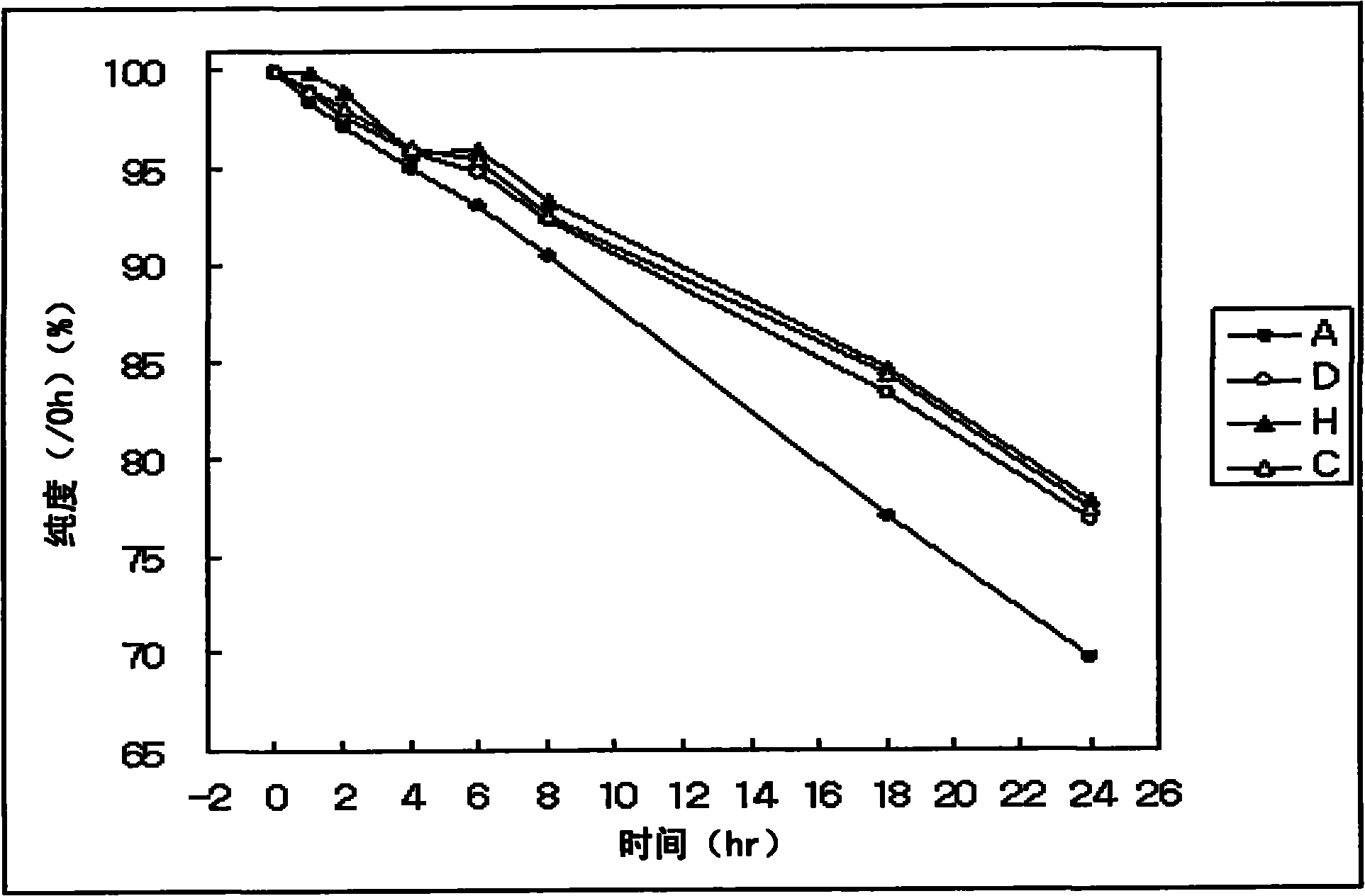
[0056] Example 1. Plasma Stability of Exendin-4 Derivatives
[0057] To determine the plasma stability of exendin-4 derivatives, each of native exendin-4 and exendin-4 derivatives was exposed to plasma and the undenatured exendin-4 derivatives were determined by reversed-phase HPLC. The amount of protein remaining for denaturation testing dependent on exposure time.
[0058] In this experiment, for the analysis of plasma-exposed samples, the pooled plasma samples were deproteinized prior to analysis.
[0059] Natural exendin-4, deamino-histidyl-exendin-4 (DA-exendin-4), β-hydroxyimidazopropionyl-exendin- 4(HY-Exendin-4), β-Carboxyimidazopropionyl-Exendin-4(CX-Exendin-4), Dimethyl-Histidyl-Exendin Exendin-4 (DM-exendin-4) and imidazoacetyl-exendin-4 (CA-exendin-4) were prepared at a concentration of 1 mg / ml, respectively. Take 200 μl of each exendin-4 derivative sample and mix with 200 μl rat serum, and react at 37°C. At each sampling time, each sample is 0 hour, 1 hour, 2 h...
Embodiment 2
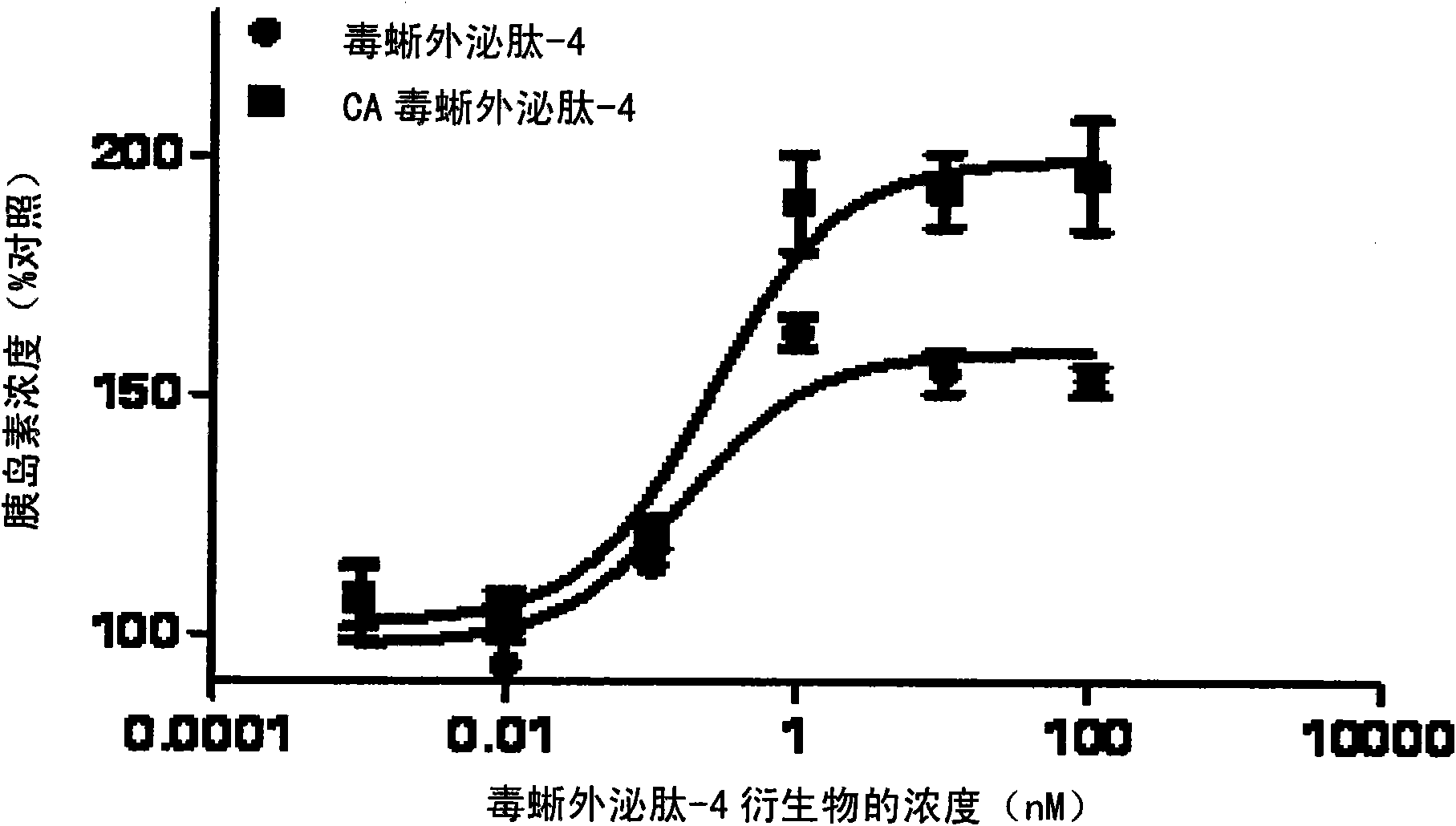
[0063] Example 2. Measuring the In Vitro Activity of Exendin-4 Derivatives
[0064] To determine the potency of exendin-4 derivatives including desamino-histidyl-exendin-4, their in vitro cell viability was tested. Natural exendin-4 and exendin-4 derivatives were synthesized by American Peptide Corporation. Insulinoma cells or islets, which are commonly used to measure GLP-1 activity in vitro, were isolated and analyzed for changes in cAMP production following GLP-1 treatment.
[0065] In this experiment, in vitro activity was measured using RIN-m5F (ATCC CRL-11605), which is known to be a rat insulinoma cell and has a GLP-1 receptor (and thus is commonly used to measure GLP-1 in vitro activity). RIN was treated with different concentrations of GLP-1, native exendin-4, and exendin-4 derivatives including N-terminal-α-deamino-histidyl-exendin-4 - m5F cells, measure cAMP production due to test material to determine EC 50 value.
[0066] Table 1
[0067] test materi...
Embodiment 3
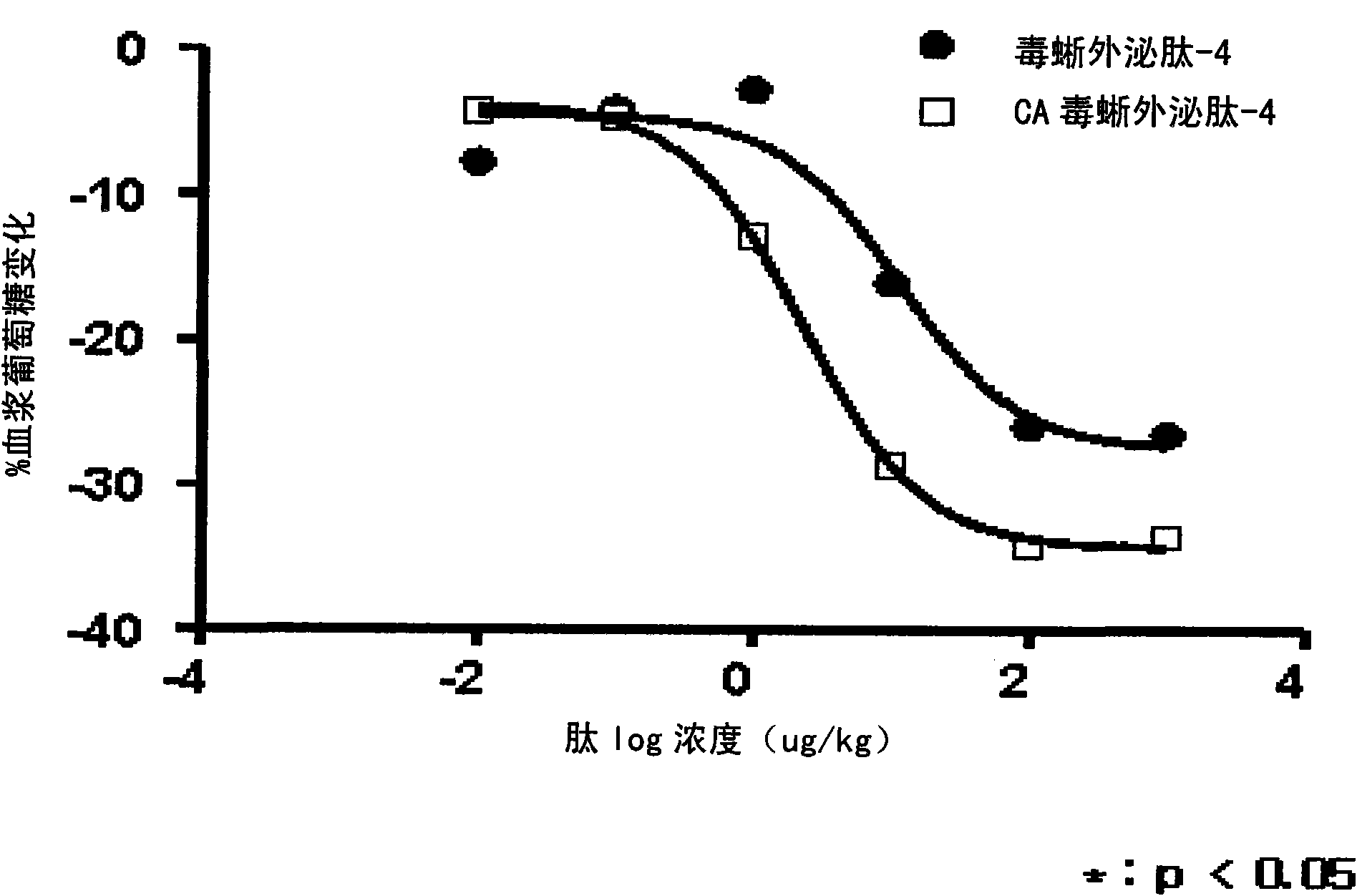
[0068] Example 3. Measuring the insulin-releasing activity of exendin-4 derivatives
[0069] The insulinotropic activity of exendin-4 derivatives was compared in RINm5F cells. Thaw RINm5F cells, subculture at least once, and then in 1x10 5 Densities of cells / well were seeded into 96-well plates with medium containing FBS (Gibco, #11082). Then in 5% CO 2 Cells were incubated for 48 hours at 37°C in an incubator. The medium was replaced with fresh medium containing 0.5% FBS, followed by incubation for 1 hour. Each of CA-exendin-4 and exendin-4 (byetta, Amylin) was diluted with a medium containing 0.5% FBS and glucose to obtain a concentration of 10 nM to 0.001 nM. In addition to exendin samples, diluted solutions were prepared and used as controls. Remove the culture medium of RINm5F cells, add the prepared samples, and then in 5% CO 2 Incubate at 37°C for 1 hour in an incubator. Then, the medium in each well was recovered. Measure the insulin concentration in the recove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com