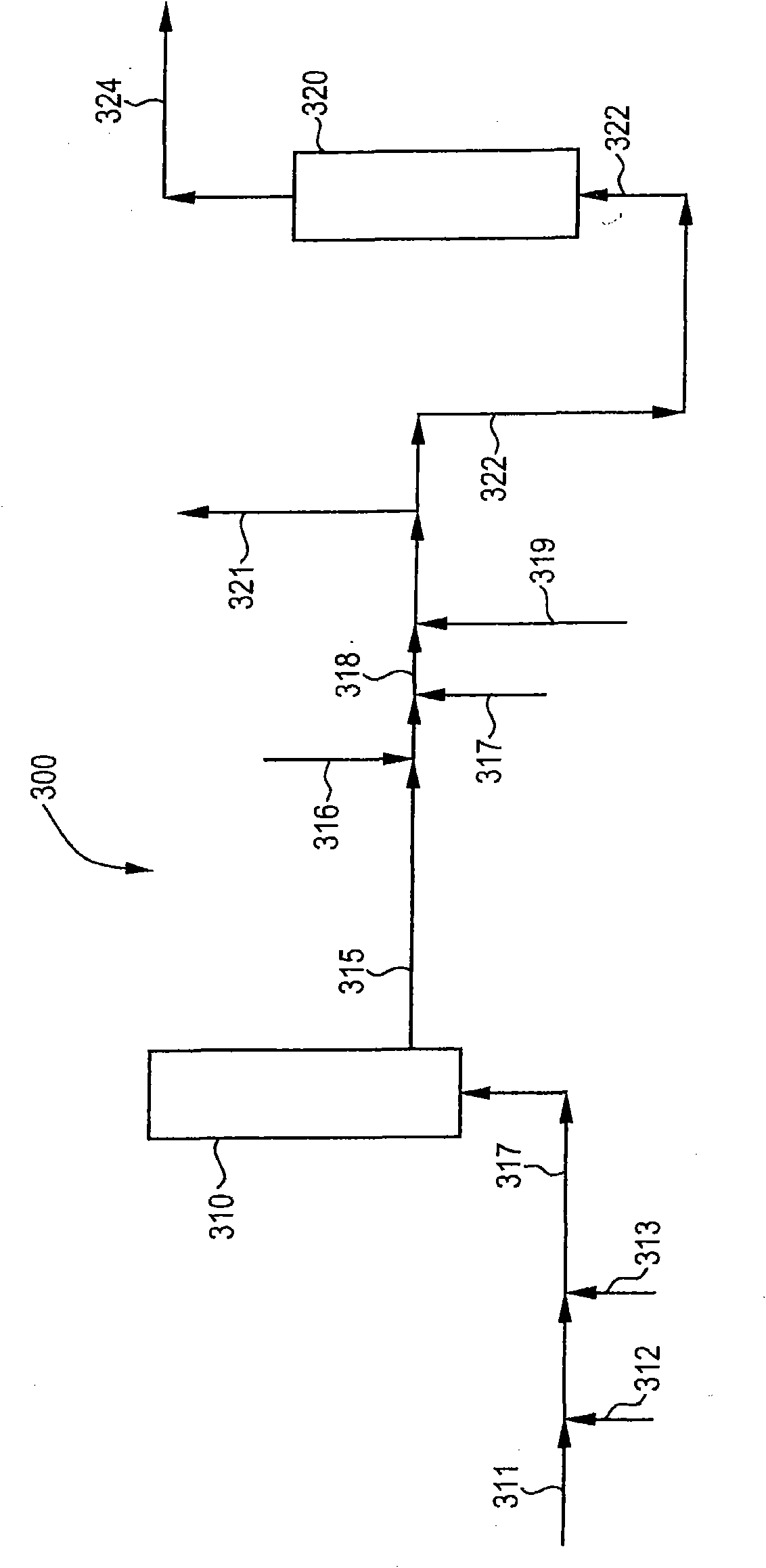
Total organic carbon (toc) reduction in brine via chlorinolysis
A technology of total organic carbon and total organic carbon content, applied in the direction of hypochlorite, chemical instruments and methods, hypochlorous acid, etc., can solve the problems of destroying organic compounds, harmful deposition of sodium chloride, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
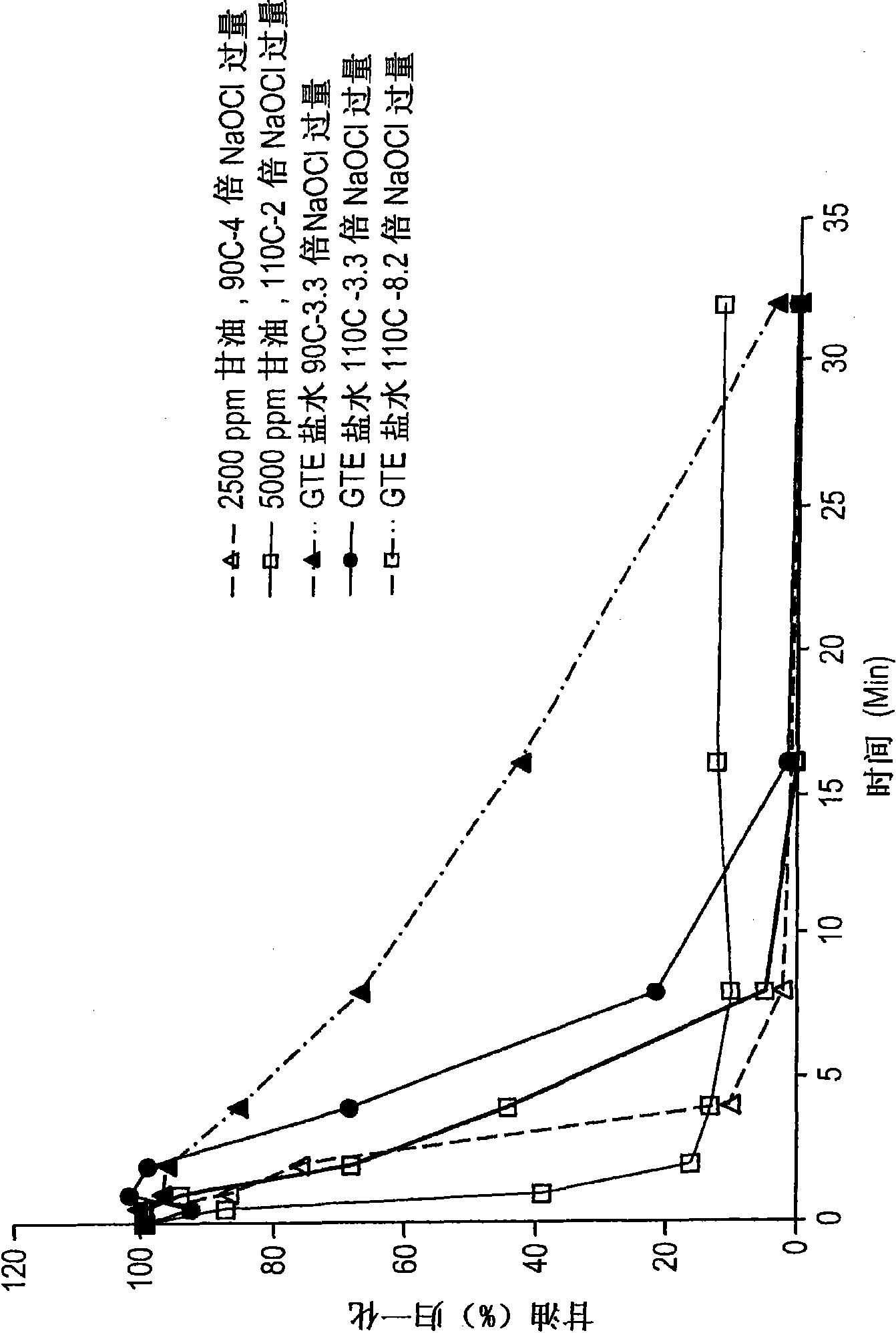
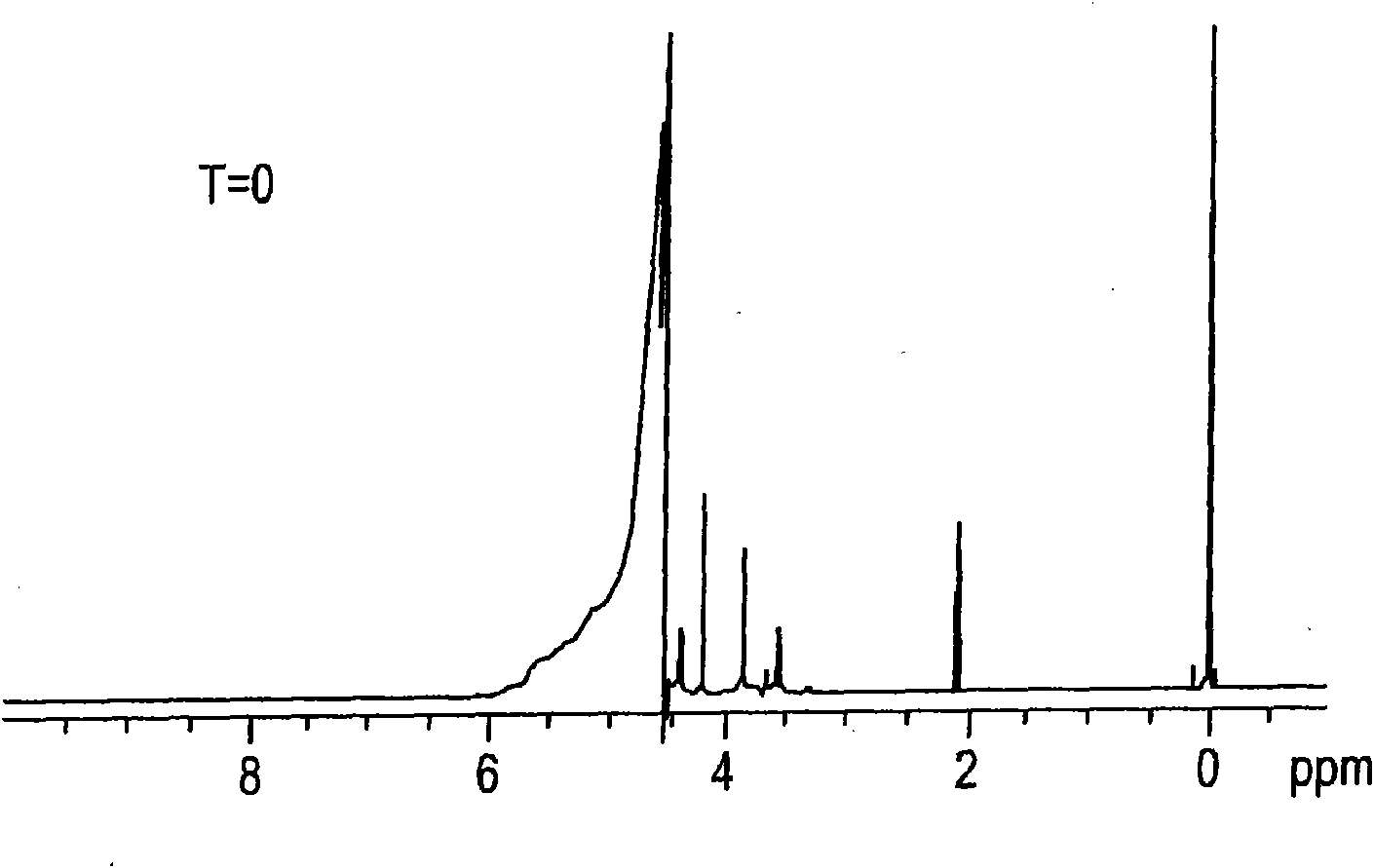
[0068] Destruction in the brine by-product stream (GTE brine) from the production of epichlorohydrin from glycerol is carried out at low or acidic pH from about 3.5 to about 5.5, and under chlorolysis conditions at high or alkaline pH from about 11.8 to about 8.5 Small-scale testing (proof) of principle laboratory experiments on organic compounds. Proof of Principle and Kinetic Studies Experiments are performed using about 1 to about 2 grams of sample in NMR tubes or reaction vials. The samples tested were pure glycerin dissolved in water, or GTE brine with a total organic carbon (TOC) content of about 1470 ppm and an initial pH of about 11.8. The sodium chloride content of the GTE brine is about 23% by weight. Synthetic glycerol samples or GTE saline samples were heated at a temperature ranging from about 90°C to about 100°C with an excess of bleach, which was about 6.5% by weight sodium hypochlorite in water, and glycerol destruction was detected by NMR. The samples tested...
Embodiment 2
[0076] Following the proof of principle test in Example 1, experiments were performed on a larger scale and in addition to monitoring glycerol destruction by NMR, total organic carbon (TOC) was monitored in chlorolysis reactions under acidic or low pH conditions. The brine by-product stream subjected to chlorination is a brine by-product stream from the production of epichlorohydrin from glycerol (GTE brine) having a TOC content of about 1470 ppm and about 23 wt% chlorine based on the weight of the GTE brine sodium chloride content, and a pH of about 9. A 133 g sample of GTE brine was mixed in a flask with approximately 66 g of commercial bleach. Commercially available bleach has a sodium hypochlorite content of about 6.5% by weight, with the balance being water.
[0077] By diluting the GTE brine with bleach, the calculated TOC content in the mixture of GTE brine and bleach was about 982 ppm. Based on calculations assuming all TOC is glycerol, the amount of glycerol in the ...
Embodiment 3
[0081] Following the proof of principle test in Example 1, experiments were performed on a larger scale and, in addition to monitoring glycerol destruction by NMR, also monitored total organic carbon (TOC) in chlorolysis reactions under alkaline or high pH conditions. ). The brine by-product stream subjected to chlorination is a brine by-product stream from the production of epichlorohydrin from glycerol (GTE brine) having a TOC content of about 1470 ppm and about 23 wt% chlorine based on the weight of the GTE brine sodium chloride content, and a pH of about 11.8. A 133 g sample of GTE brine was mixed with approximately 56 g of commercial bleach in a flask.
[0082] Commercially available bleach has a sodium hypochlorite content of about 6.5% by weight, with the balance being water.
[0083] By diluting the GTE brine with bleach, the calculated TOC content in the mixture of GTE brine and bleach was about 1040 ppm. Based on calculations assuming all of the TOC is glycerol, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com