Synthesis and production process of anti-cancer medicament bis(4-fluorobenzyl) trithioether and organic trithioether derivative
A technology of lepisol and process flow, which is applied in the field of synthesis and production technology of aromatic ring-substituted organic trisulfide derivatives, and can solve problems such as complex synthetic routes, unstable synthesis formation, difficult separation and purification of trisulfide derivatives, etc. Achieve the effect of improving the separation and purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0158] Specific embodiment 1 Detailed production process flow of fluperazine synthesis
[0159] Starting materials and reagents:
[0160] The starting materials and reagents required for the synthesis of fluloperidol are listed in Table 1.
[0161] Table 1. Starting materials and reagents
[0162]
[0163] Detailed process flow:
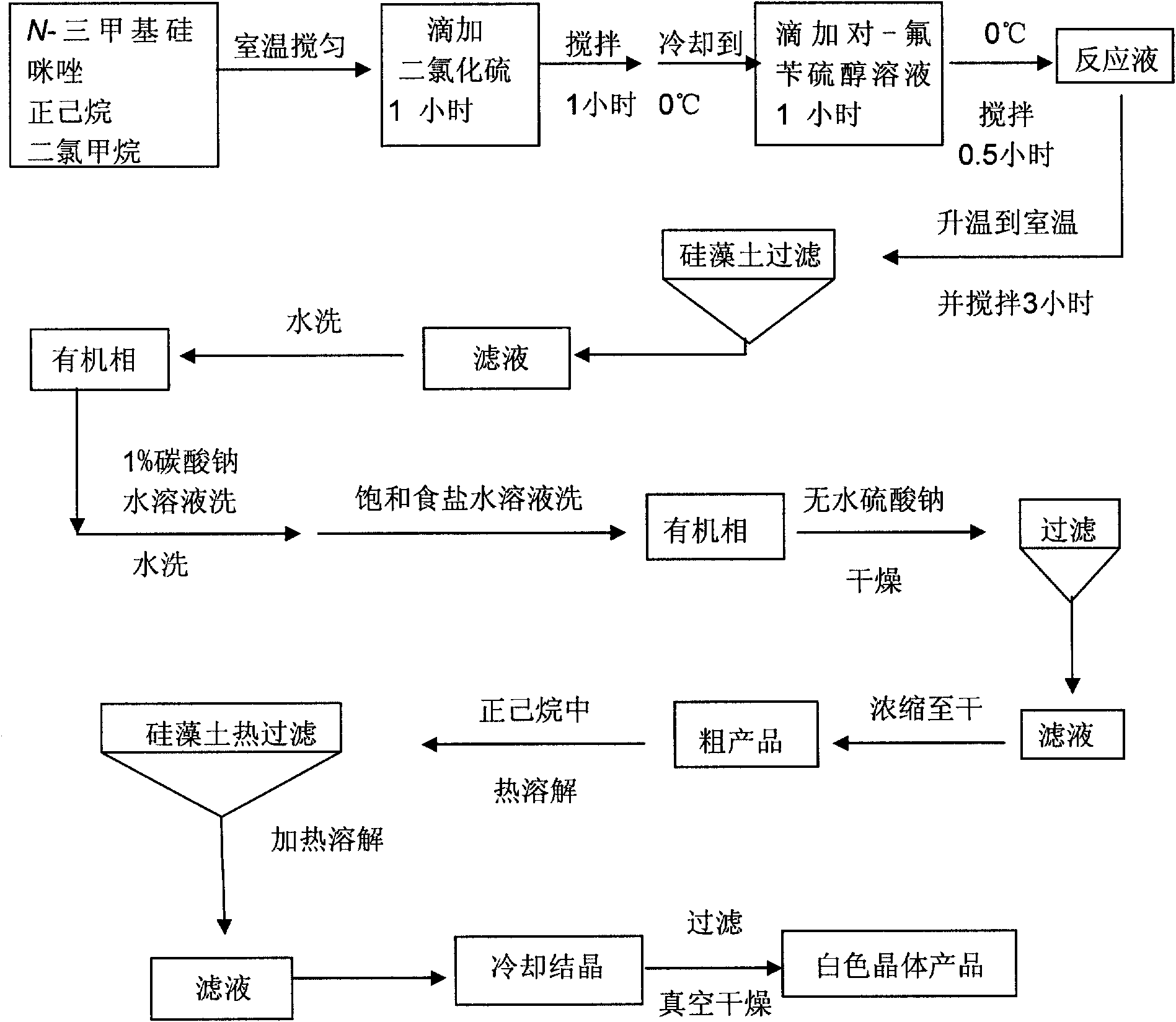
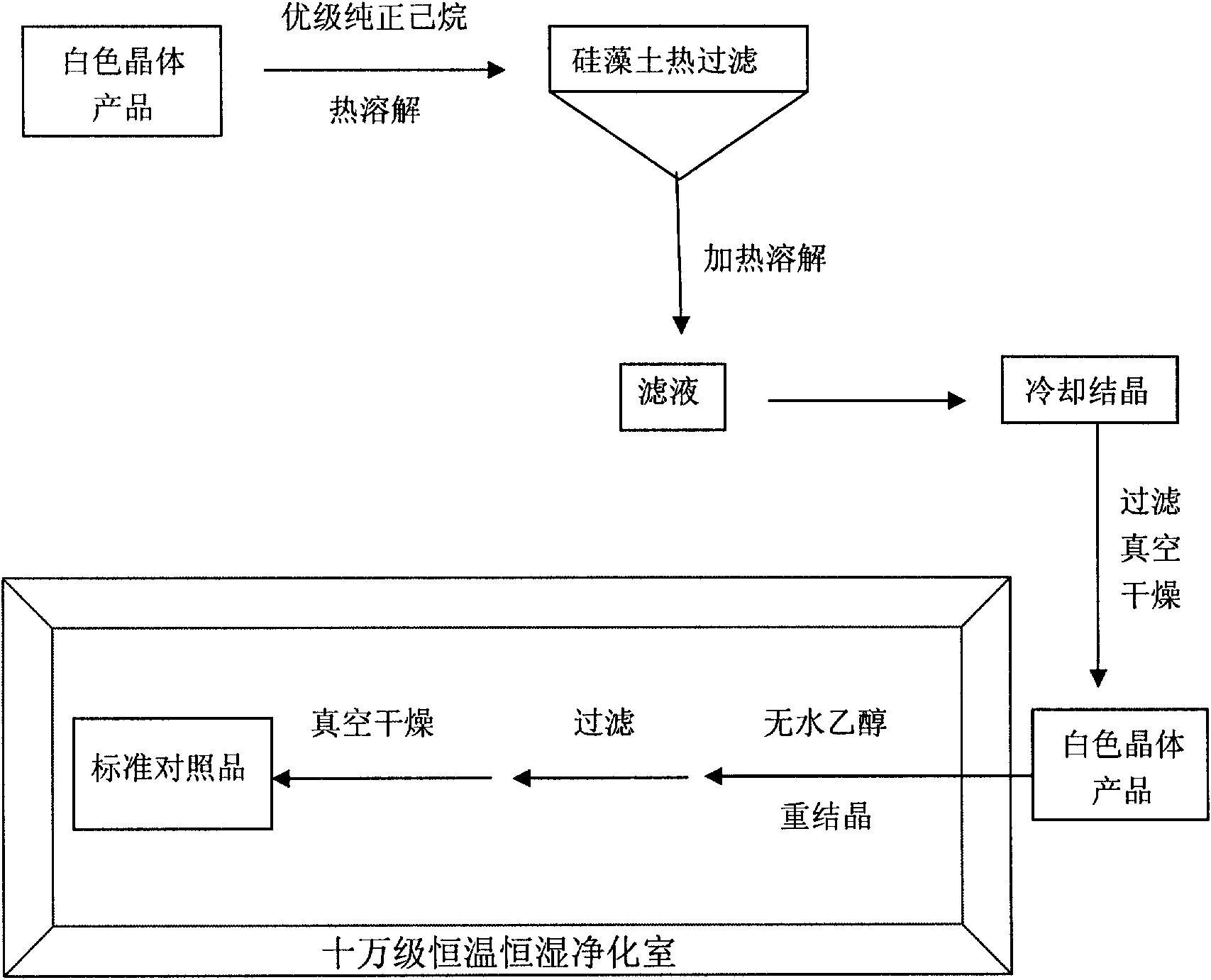
[0164] 1) Install a mechanical stirrer and a dropping funnel in a 5L (or 10L) three-neck round-bottom flask (or S212B double-layer glass reactor with variable frequency and constant speed) and feed nitrogen. respectively will go through 3 1500ml of n-hexane and 500ml of dichloromethane dried over molecular sieves were added to the reaction flask, and 643.5ml (4.386mol) of N-trimethylsilimidazole was added under stirring at room temperature. After the solution was evenly stirred, 139.4 ml (2.194 mol) of sulfur dichloride was slowly added dropwise at room temperature under the protection of nitrogen and constant stirring for 1 hour. A large am...
specific Embodiment approach 2
[0172] Specific embodiment 2 Detailed production process flow of fluperazine synthesis
[0173] Starting materials and reagents:
[0174] The starting materials and reagents required for the synthesis of fluloperidol are listed in Table 2.
[0175] Table 2. Starting materials and reagents
[0176]
[0177] Detailed process flow:
[0178] 1) Install a mechanical stirrer and a dropping funnel in a 50L reactor and feed nitrogen into it, and pass through 3 18800ml of n-hexane and 5300ml of dichloromethane dried by molecular sieves were added to the reaction flask, and 4158ml (27.49mol) of N-trimethylsilimidazole was added under stirring at room temperature. After the solution was evenly stirred, 852 ml (13.41 mol) of sulfur dichloride was slowly added dropwise at room temperature under nitrogen protection and constant stirring for one hour. A large amount of white solid formed during the dropwise addition. After the addition was complete, the reaction mixture was stirred ...
specific Embodiment approach 3
[0186] Specific embodiment 3 Detailed production process for the synthesis of fluperazine
[0187] Starting materials and reagents:
[0188] The starting materials and reagents required for the synthesis of fluloperidol are listed in Table 3.
[0189] Table 3. Starting materials and reagents
[0190]
[0191] Process flow:
[0192] 1) Install a mechanical stirrer and a dropping funnel in a 100L reactor and feed nitrogen into it, and pass through 3 36000ml of n-hexane and 11000ml of dichloromethane dried by molecular sieves were added to the reaction flask, and 8880ml (62.52mol) of N-trimethylsilimidazole was added under stirring at room temperature. After the solution was evenly stirred, 1775 ml (27.98 mol) of sulfur dichloride was slowly added dropwise at room temperature under nitrogen protection and constant stirring, and the dropping time was one hour. A large amount of white solid formed during the dropwise addition. After the addition was complete, the reaction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com