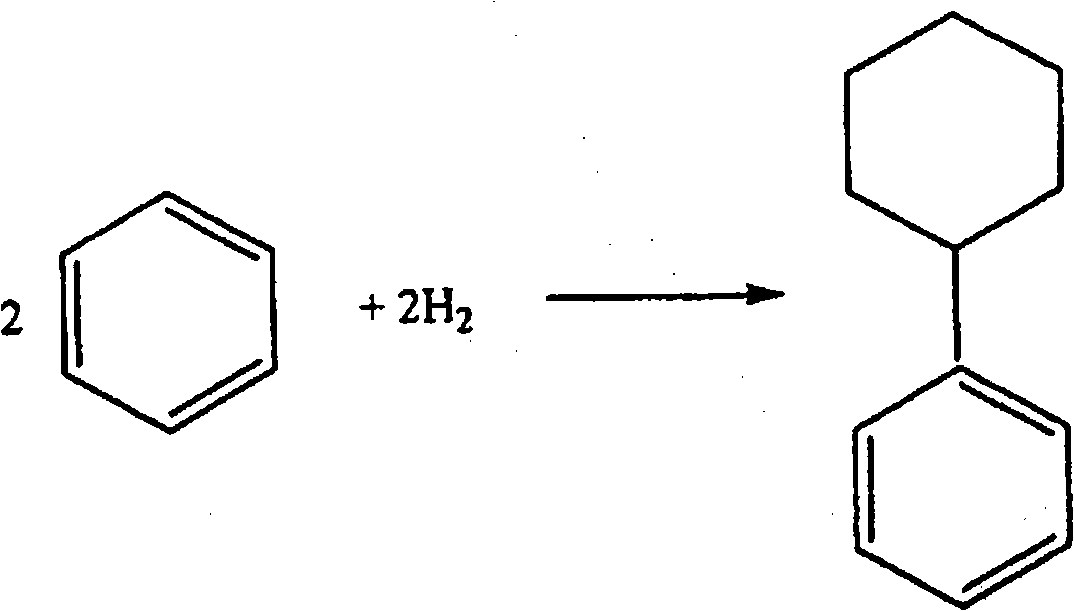
Process for producing cyclohexylbenzene
A technology of cyclohexylbenzene and cyclohexanone, applied in the field of preparing cyclohexylbenzene, can solve problems such as the increase of the cost of propylene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0059]To illustrate the importance of the location of the hydrogenation metal in hydroalkylation, Table 1 below compares the presence of the same amount of palladium, but with palladium on a molecular sieve (catalyst A) or on an inorganic oxide support (catalyst B). Performance of two catalysts A and B. Both catalysts contained 0.006 g of Pd and 1.6 g of MCM-49, with Catalyst A containing 0.4 g gamma alumina support and Catalyst B containing 2.0 g gamma alumina support.
[0060] Catalyst A was prepared by depositing palladium onto MCM-49 zeolite by incipient wetness technique. The gamma alumina was then added to the Pd-impregnated MCM-49 to form a mixture. The mixture was then pelletized using a hand press at 136,000 kPaa for 60 seconds to form pellets. The pellets were then sized to pass through 0.841 mm openings but not 0.250 mm openings (20 / 60 mesh pellets) and tested for benzene hydroalkylation performance. With Catalyst A, all palladium was on molecular sieves.
[006...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com