Method for preparing Fe3O4@ZrO(OH)2 magnetic nano-adsorbing material for high-efficient fluoride removal from drinking water
A magnetic nanometer and adsorption material technology, applied in chemical instruments and methods, adsorption water/sewage treatment, water pollutants, etc., can solve the problems of secondary pollution, fast attenuation of adsorption performance, low fluorine removal efficiency, etc. Fast, short adsorption path, simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
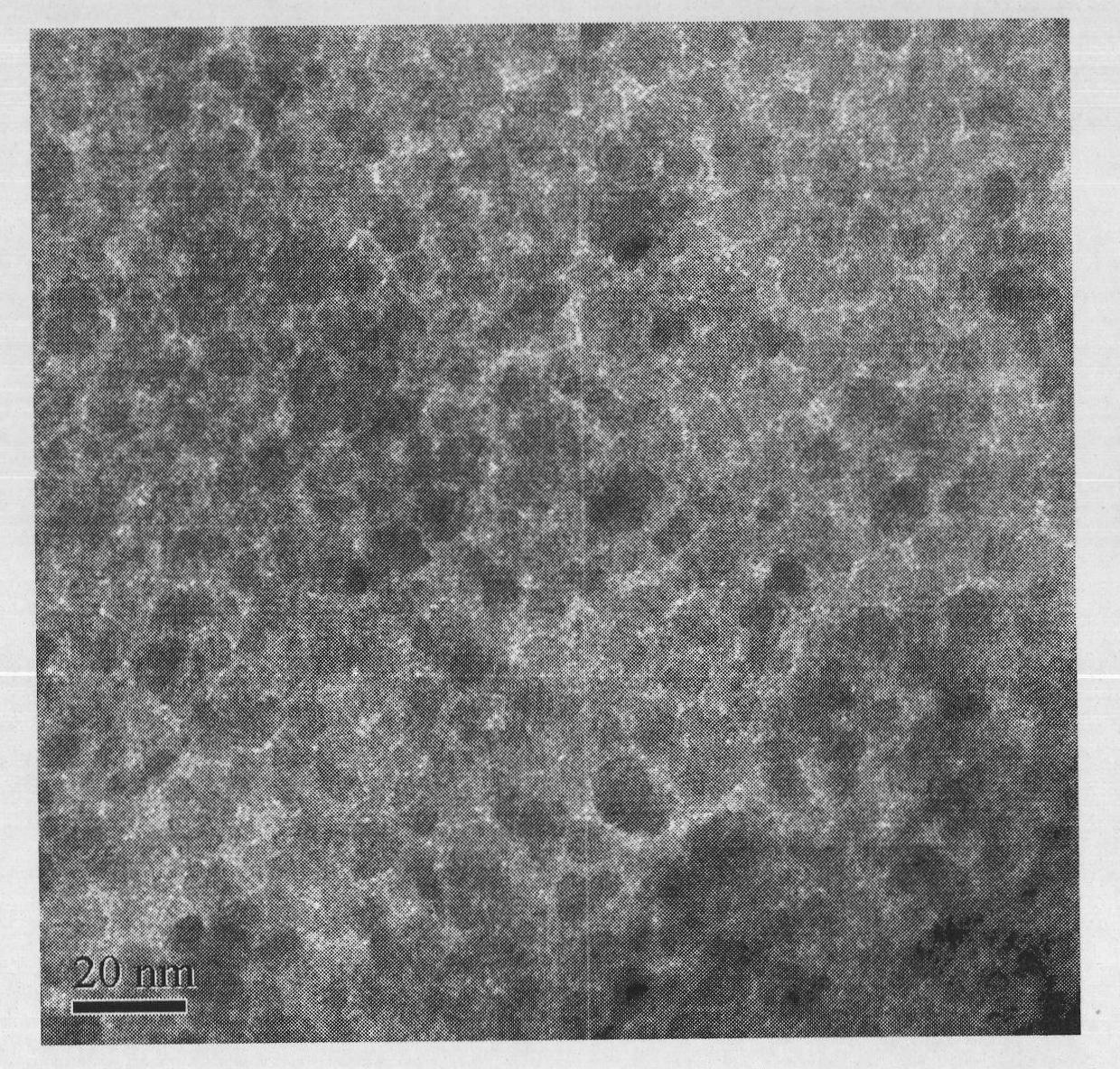
[0013] Preparation of Magnetic Nano Fe by Co-precipitation 3 o 4 Microspheres and Magnetic NanoFe 3 o 4 @ZrO(OH) 2 Composite microspheres. Weigh 27.000gFeCl 3 ·6H 2 O and 15.000 g FeSO 4 ·7H 2 Mix O in a three-neck flask, add 50mL of deionized water, blow nitrogen for 2-3min, put it into a constant temperature water bath at 85°C under the condition of mechanical stirring, add 150mL of 2mol / L NaOH dropwise, and react for two hours Magnetic Fe can be obtained by washing with an external magnetic field 3 o 4 Microspheres.
[0014] Weigh 3.000g of prepared magnetic nano-Fe 3 o 4 Add microspheres and 50mL deionized water into a three-necked flask, put it into a constant temperature water bath at 85°C under the condition of mechanical stirring, first add 22.810g ZrOCl dropwise 2 ·8H 2 After the solution of O, add 2mol / L NaOH solution dropwise until the color of the solution changes from black to dark yellow. After two hours of reaction, use an external magnetic field t...
Embodiment 2
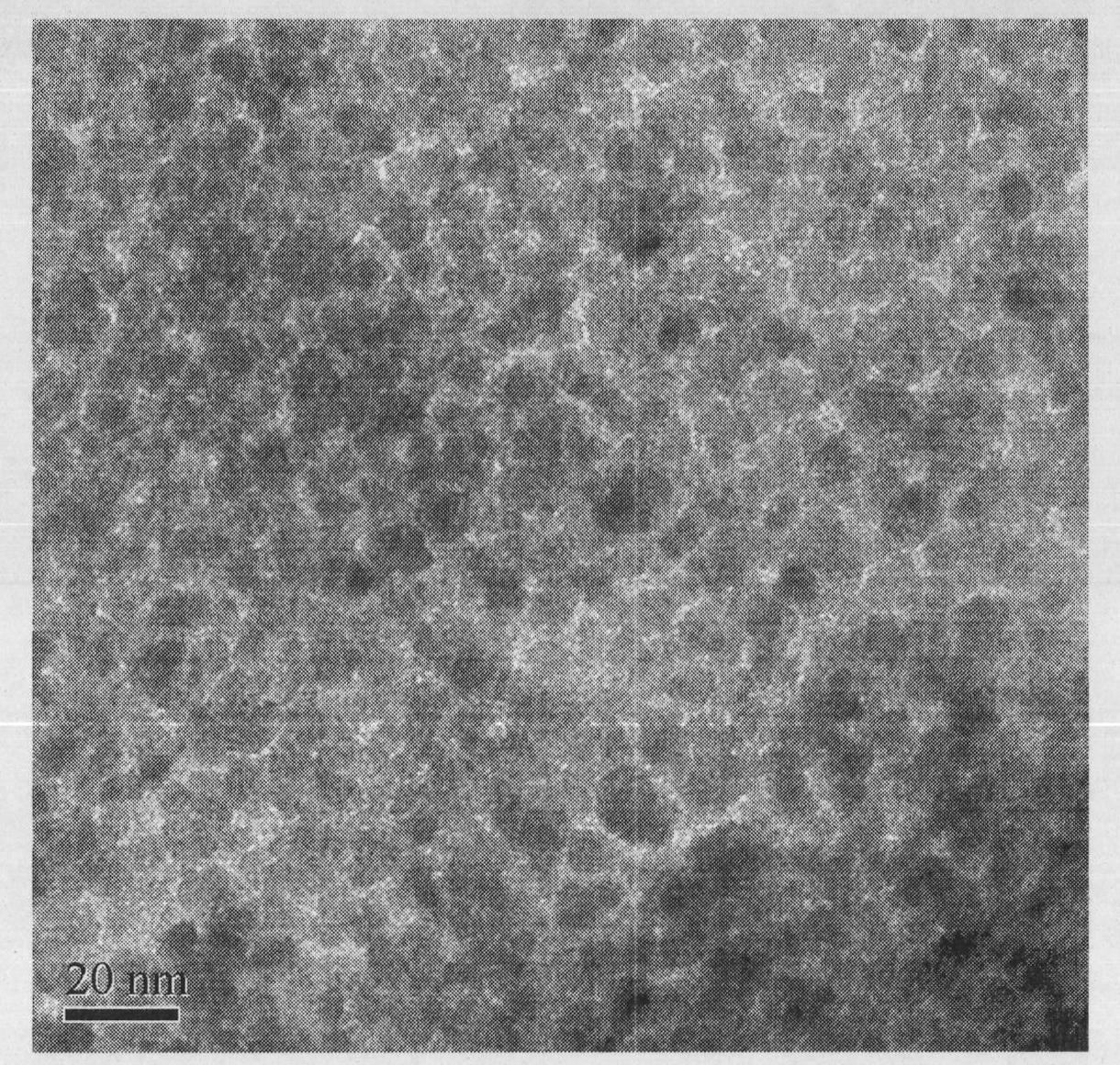
[0016] Take by weighing 3.000g magnetic nanometer Fe that above-mentioned example prepares 3 o 4 Add microspheres and 50mL deionized water into a three-necked flask, put it into a constant temperature water bath at 85°C under the condition of mechanical stirring, first add 11.405g ZrOCl dropwise 2 ·8H 2 After the solution of O, add 2mol / L NaOH solution dropwise until the color of the solution changes from black to dark yellow. After reacting for 1 hour, use an external magnetic field to separate and wash to obtain magnetic nano-Fe 3 o 4 with ZrO(OH) 2 Magnetic nano-Fe with a mass ratio of 3:5 3 o 4 @ZrO(OH) 2 Composite microspheres.
Embodiment 3
[0018] Take by weighing 3.000g example 1 prepared magnetic nano-Fe 3 o 4 Add the microspheres and 50mL deionized water into the three-necked flask, and put it into the magnetic nano-Fe in the constant temperature water bath at 85°C under the condition of mechanical stirring. 3 o 4 Add microspheres and 50mL deionized water into a three-necked flask, put it into a constant temperature water bath at 85°C under the condition of mechanical stirring, first add 45.621g ZrOCl dropwise 2 ·8H 2 After the solution of O, add 2mol / L NaOH solution dropwise until the color of the solution changes from black to dark yellow. After reacting for 3 hours, use an external magnetic field to separate and wash to obtain magnetic nano-Fe 3 o 4 with ZrO(OH) 2 Magnetic nano-Fe with a mass ratio of 3:20 3 o 4 @ZrO(OH) 2 Composite microspheres.
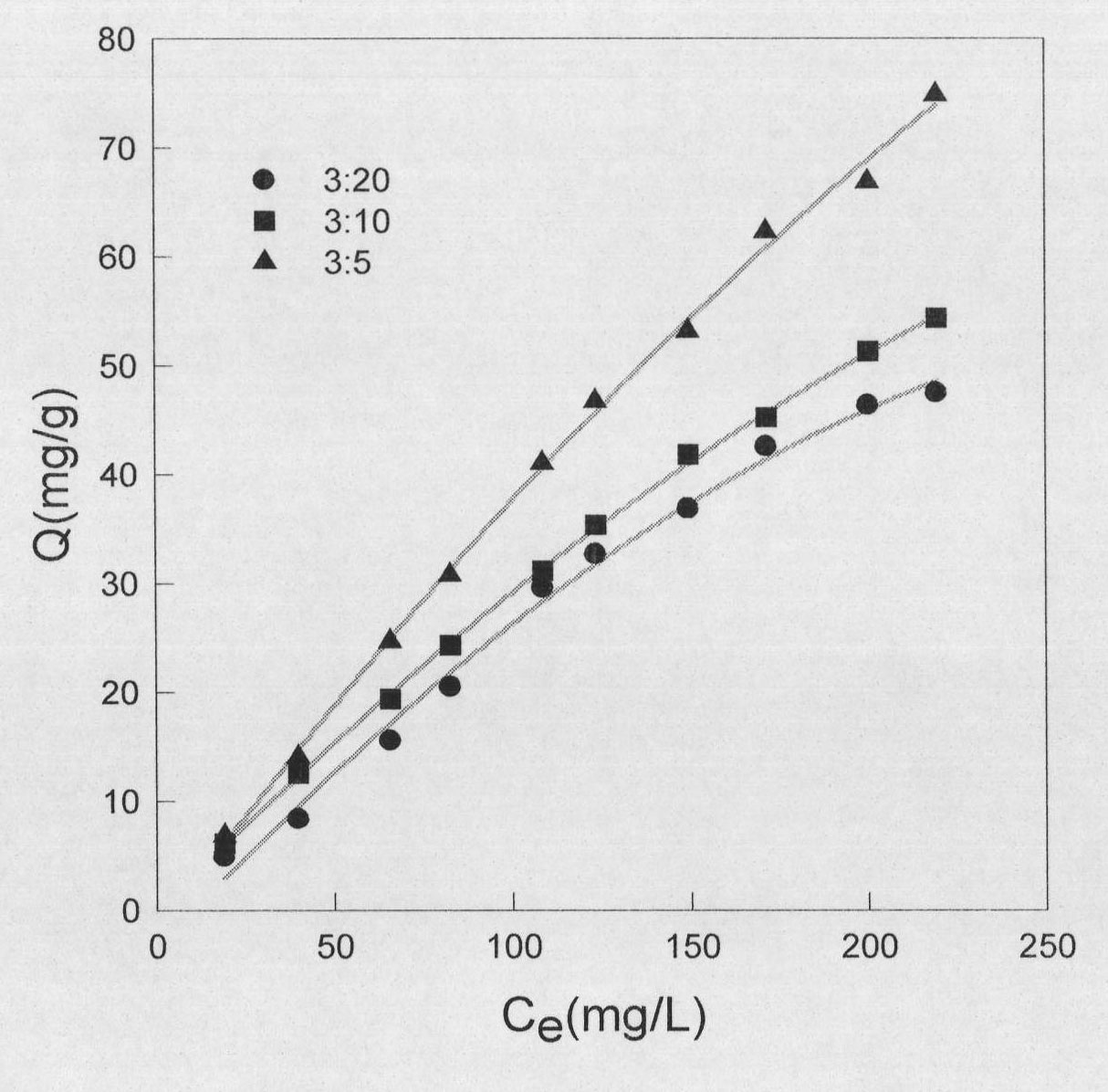
[0019] Obtain magnetic Fe as shown in Figure 2 by embodiment 1,2,3 3 o 4 @ZrO(OH) 2 Adsorption isotherm of fluoride ions by composite nano-materials ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com