Convenient synthesis method of methylprotodioscin
A technology of methyl raw potato and synthesis method, applied in the direction of steroids, organic chemistry, etc., to achieve the effect of abundant resources and low price
Inactive Publication Date: 2010-08-25
YUNNAN UNIV
View PDF0 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
At present, the synthesis of methyl protodiosgenin has not been reported. How to synthesize methyl protodiosgenin concisely and efficiently using high-yield and cheap raw materials has attracted widespread attention from synthetic workers.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
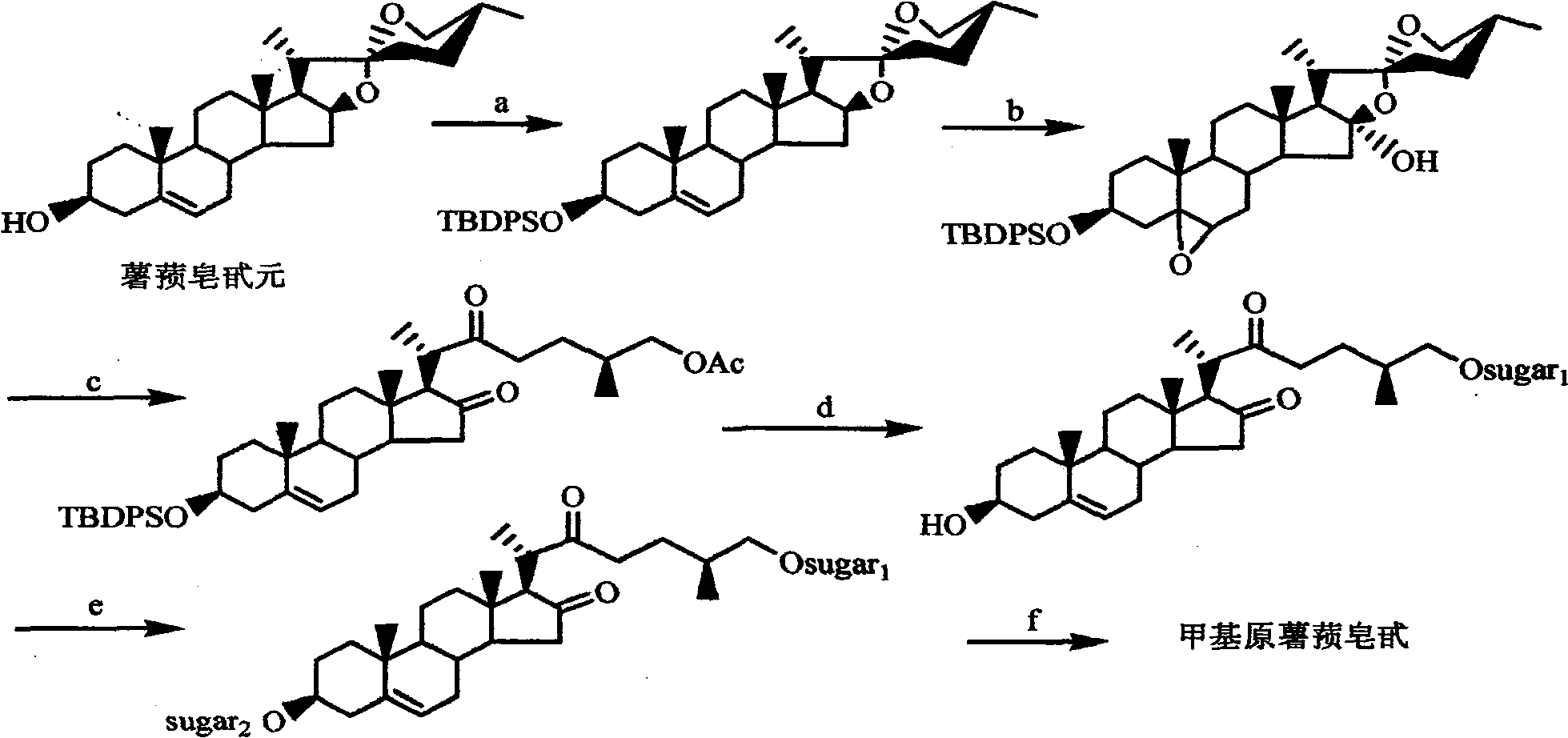
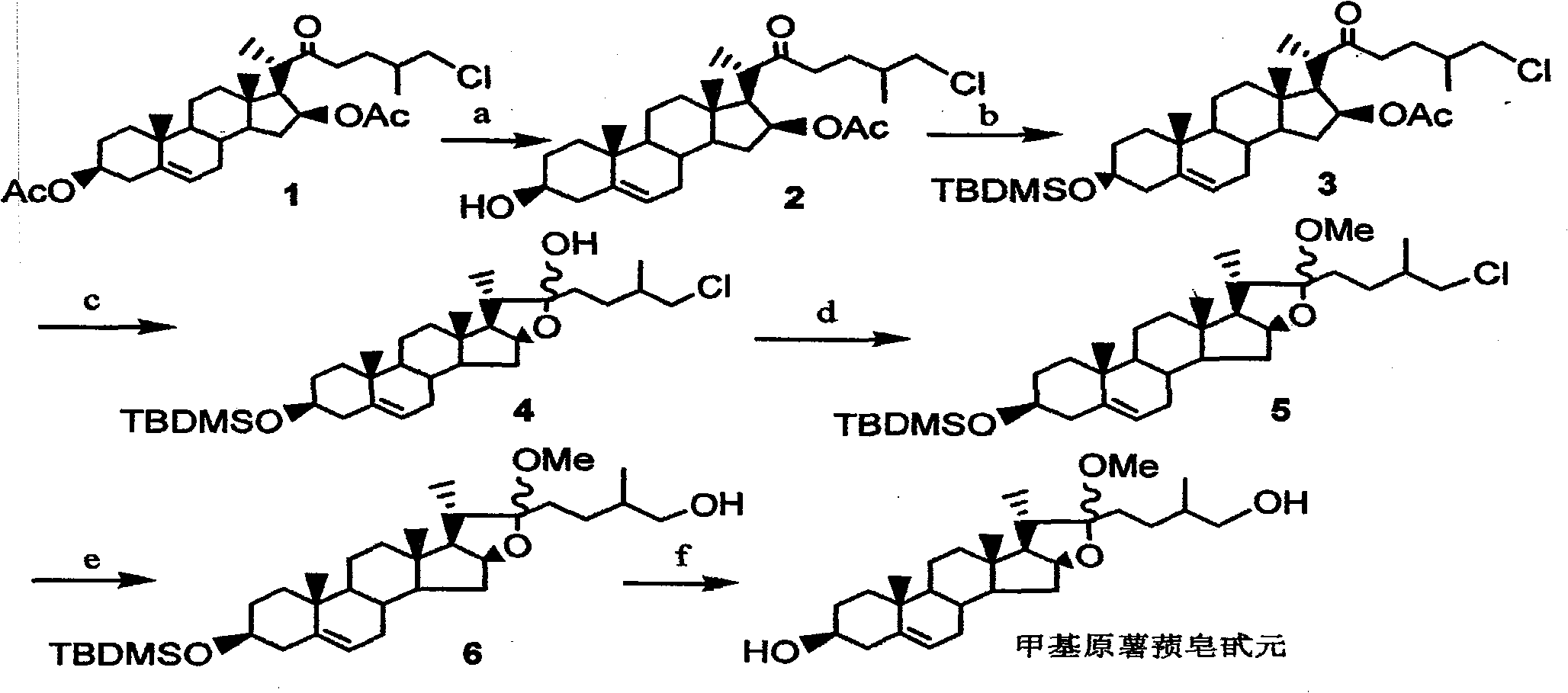
A convenient synthesis method of methylprotodioscin belongs to the organic synthesis technical field. The method comprises the following steps: using the rich and cheap E / F full-ring-opening product of protodioscin, namely 26-choro-3beta,16beta-diacetoxy-22-one-5-cholestene as raw material to perform selective alcoholysis of C3-OAc and protection of C3-OH, then in THF / MeOH mixed solution, using the high selectivity of weak base K2CO3 to close E-ring to form C22-OH, then using methyl iodide to perform methylation to C22-OH, and finally performing a substitution reaction and deprotection of C3-OH. The synthesis of methylprotodioscin is realized in a concise and atom economy manner. The raw material and reagents used in the invention are cheap, the operation is simple, the reaction conditions are mild, the product is easy to separate and purify, the yield is high, especially by using the selective ring-opening reaction and the methylation reaction using the active methylating agent methyl iodide, the construction of furan ring in methylprotodioscin and the methylation of C22-OH can be effectively realized, and by attaching the same or different sugar chains to the C3 and C26 of the methylprotodioscin molecule, methylprotodioscin and multiple analogs can be synthesized. Therefore, the method provides a new synthetic approach for methylprotodioscin and other similar saponins.
Description
A Simple Synthesis Method of Methylprotodiosgenin technical field The invention belongs to the technical field of organic synthesis. Background technique Steroidal saponins are a class of oligoglycosides that combine sterol compounds with 27 carbon atoms as aglycones and oligosaccharides. They are widely distributed in nature, mainly concentrated in plants such as Liliaceae, Dioscoreaceae and Agaveaceae, and most concentrated in the genus Dioscorea and Liliaceae. From the structure, it can be divided into spirosterane type, furostane type and cholestane type. Furanostane type saponins are an important class of steroidal compounds, and most of them are disaccharide chain saponins in structure, usually in the aglycon Both C3 and C26 positions are connected with sugar chains, and its representative compound is methylprotodioscin (methylprotodioscin.fig.1), which was first isolated from fresh roots of Dioscoreaceae in 1974. Methylprotodioscin is usually Together with diosgen...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07J71/00
Inventor 朱洪友雷泽胡大伟程水连木晓云温晓江付正启
Owner YUNNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com