Veterinary compound gentamycin sulfate injection and preparation method thereof
A technology of gentamicin sulfate and injection, which is applied in the direction of medical preparations containing active ingredients, blood diseases, pharmaceutical formulas, etc., can solve problems such as incomplete curative effects, and achieve broad antibacterial spectrum, good antibacterial effect, and ease The effect of superficial symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A compound gentamycin sulfate injection for veterinary use contains the following components, all in parts by weight:
[0032] 4 parts of gentamicin sulfate, 2 parts of ofloxacin, 1.5 parts of trimethoprim, 30 parts of analgin, 0.15 parts of sodium bisulfite, 0.04 parts of disodium edetate, N, N-di 20 parts of methyl formamide, 15 parts of propylene glycol, 3.5 parts of glacial acetic acid; 40 parts of water for injection.
[0033] The preparation method of aforementioned veterinary compound gentamicin sulfate injection, the steps are as follows:
[0034] 1) After dissolving 1.5 parts of trimethoprim with 20 parts of N,N-dimethylformamide, a trimethoprim solution was obtained;
[0035] 2) After dissolving 2 parts of ofloxacin with 2 parts of glacial acetic acid and 5 parts of water for injection, a solution of ofloxacin is obtained;
[0036] 3) Dissolve 30 parts of Sulpyrine in 25 parts of water for injection to obtain a Sulpyrine solution; dissolve 4 parts of gentami...
Embodiment 2
[0054] A compound gentamycin sulfate injection for veterinary use contains the following components, all in parts by weight:
[0055] 2 parts of gentamicin sulfate, 4 parts of ofloxacin, 2 parts of trimethoprim, 10 parts of analgin, 0.1 part of sodium bisulfite, 0.02 part of disodium edetate, N, N-di 35 parts of methyl formamide, 15 parts of propylene glycol, 6 parts of glacial acetic acid; 30 parts of water for injection.
[0056] The preparation steps were the same as in Example 1 to obtain compound gentamycin sulfate injection for veterinary use, with a pH of 3.7.
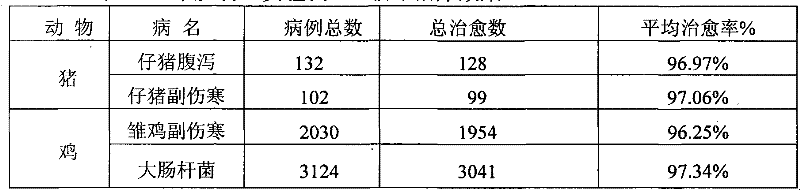
[0057] Table 3 The present invention (experimental example 2) clinical treatment effect
[0058]
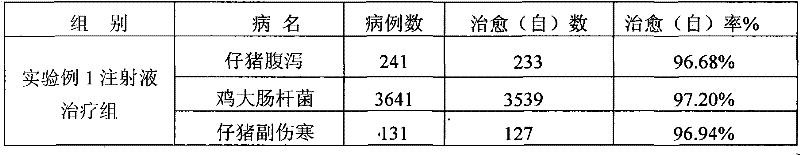
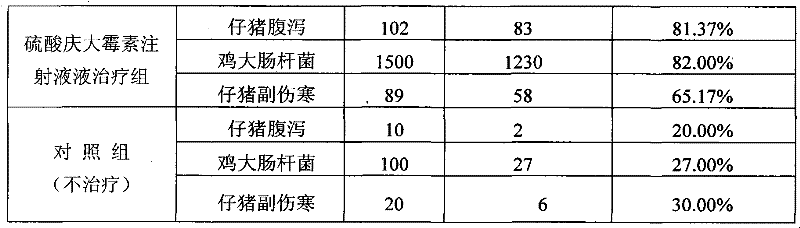
[0059] Table 4 The present invention and Gentamicin Sulfate Injection clinical treatment comparative test result
[0060]
Embodiment 3
[0062] A compound gentamycin sulfate injection for veterinary use contains the following components, all in parts by weight:
[0063] 5 parts of gentamicin sulfate, 5 parts of ofloxacin, 2.5 parts of trimethoprim, 30 parts of analgin, 0.2 parts of sodium bisulfite, 0.04 parts of disodium edetate, N, N-di 25 parts of methyl formamide, 10 parts of propylene glycol, 7.5 parts of glacial acetic acid; 25 parts of water for injection.
[0064] The preparation steps were the same as in Example 1 to obtain compound gentamycin sulfate injection for veterinary use, with a pH of 3.9.
[0065] Table 5 The present invention (experimental example 3) clinical treatment effect
[0066]
[0067] Table 6 The present invention and Gentamicin Sulfate Injection clinical treatment comparative test result
[0068]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com