Preparation process of non-PVC-soft-bag-packaged hemofiltration replacement liquid and product
A technology of hemofiltration and preparation technology, applied in the field of medicine, can solve the problems that the basic formula of hemofiltration replacement fluid cannot meet clinical requirements, cannot meet market demand, and has no double valve design, so as to prevent secondary pollution and shorten the detection cycle. Short, easy to adjust the effect of the prescription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] The embodiment of the preparation process of a kind of non-PVC soft bag packing hemofiltration replacement fluid of the present invention:
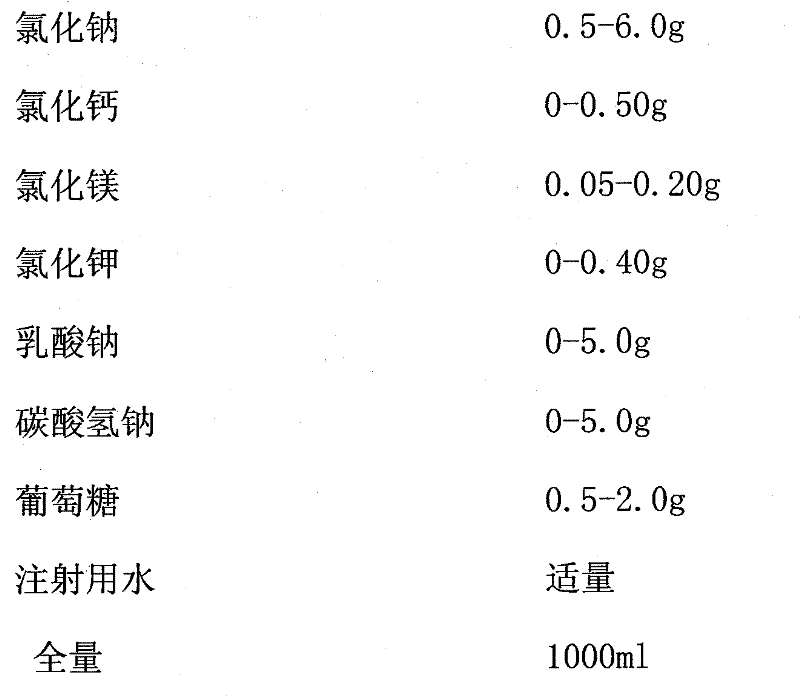
[0036] A. The ratio of raw materials by weight is:
[0037] Sodium chloride (NaCl) 0.5-6.0g
[0038] Calcium Chloride (CaCl 2 2H 2 O) 0-0.50g
[0039] Magnesium Chloride (MgCl 2 ·6H 2 O) 0.05-0.20g
[0040] Potassium chloride (KCl) 0-0.40g
[0041] Sodium Lactate (C 3 h 5 NaO 3 ) 0-5.0g
[0042] Sodium bicarbonate (NaHCO 3 ) 0-5.0g
[0043] Glucose (C 6 h 12 o 6 ·H 2 O) 0.5-2.0g
[0044] Appropriate amount of water for injection
[0045] Full volume 1000ml
[0046] B. Preparation process
[0047] a. Preparation of liquid medicine: add at least 60% of the prescribed amount of water for injection into the concentrated preparation tank, then add at least 102.0% of the prescribed amount of anhydrous glucose, calcium chloride, magnesium chloride, at least 104.0% of the prescribed amount of sodium lactate solution and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com