Low-swelling biocompatible hydrogels
A hydrogel, precursor technology, applied in medical science, prosthesis, etc., can solve problems such as inappropriate spine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1: Low swelling hydrogel formulation
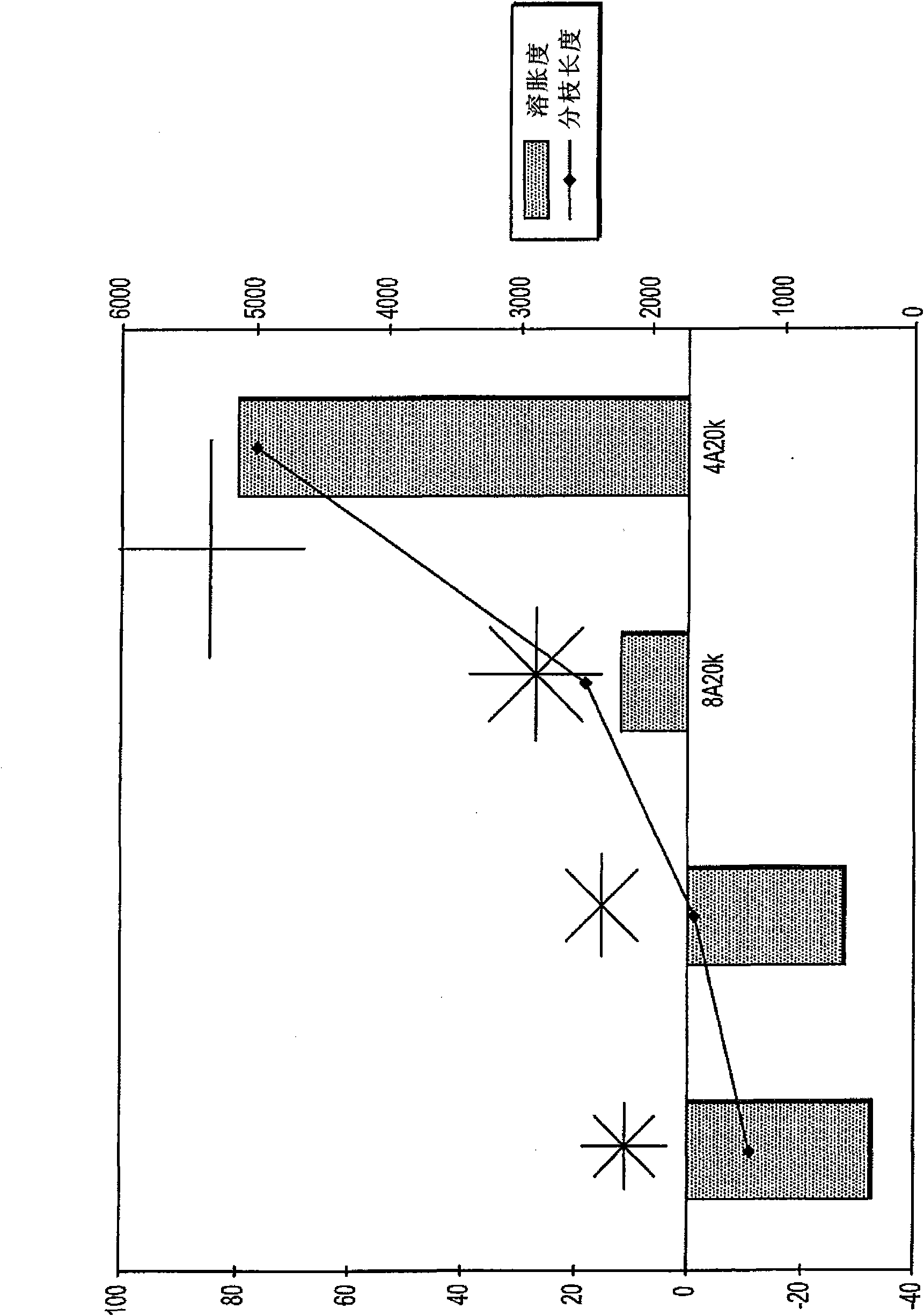
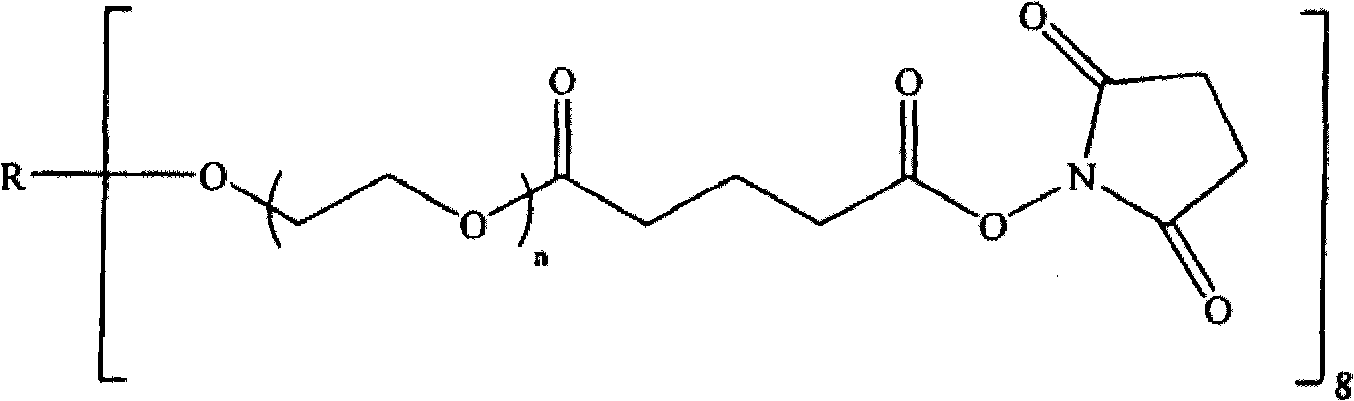
[0100] To prepare the hydrogel, trilysine containing primary amine functional groups was reacted with a branched polyethylene glycol (PEG) electrophilic precursor in a 1:1 electrophile:nucleophile stoichiometric ratio, The multi-branched polyethylene glycol (PEG) electrophilic precursor (sometimes also referred to herein as 4a20k SG) has four branches (4a), each with a total MW of about 20,000 MW of polyethylene glycol And the ends all contain succinimidyl ester electrophilic functional groups (specifically, succinimidyl glutarate, SG).
[0101] Then, instead of using a 6-arm (6a) or 8-arm (8a) precursor containing PEG arms with a total MW of approximately 10,000 (10k) or 20,000 (20k) (with functional groups at the end of each arm) In addition to the tetra-branched precursor, essentially the same hydrogels were prepared, still using the 1:1 ratio of electrophile-nucleophile functional groups.
[0102] Taking 4a20k SG as a...
Embodiment 2
[0112] Example 2: The role of the osmotic environment in swelling
[0113] Hydrogels were prepared by using 4a20k SG as described in Example 1 and exposed to physiologically buffered saline with a pH of 7.0-7.4 and an osmolarity of approximately 300 mOs or to a double strength solution of the same saline to test the effect of osmotic environment on swelling. When n=3 (hydrogel plug per molar concentration of PBS), the degree of swelling from gelation to equilibrium swelling (recorded at 24 hours) averaged 68% for normal saline and 57% for double strength saline. %. These results suggest that differences in osmotic pressure inherent in the swelling environment are not responsible for the decrease in swelling degree of hydrogels prepared with precursors with different branch lengths, as the change in swelling degree is too small to be observed when the branch length increases. explain the generally observed larger variation.
Embodiment 3
[0114] Example 3: Test of low swelling hydrogel in vivo
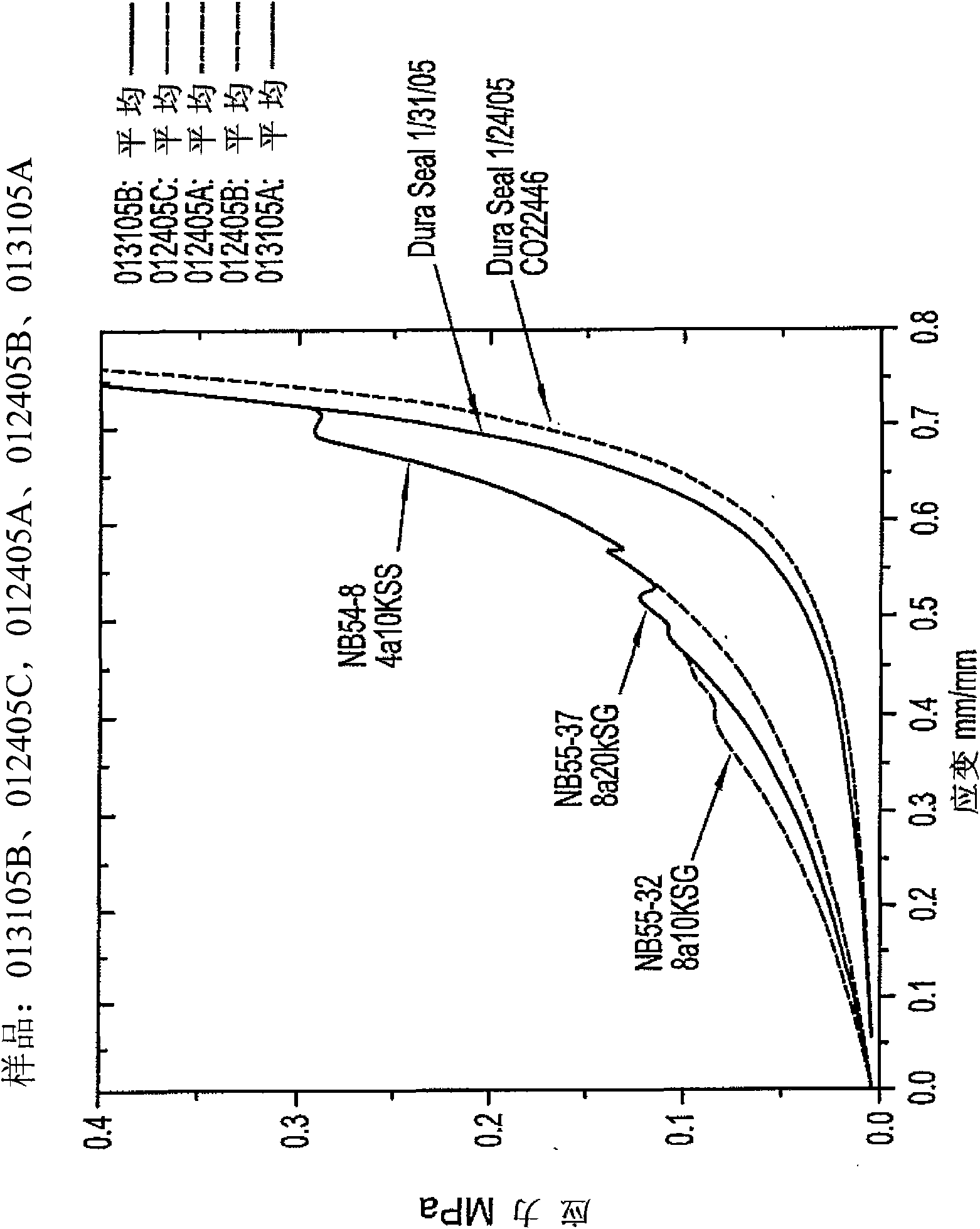
[0115] Implantation of low-swelling hydrogels in living spines. Formulation 1 was prepared by combining a trilysine precursor with an 8a15k SG (8 branched PEG precursor, each branch having a total PEG MW of approximately 15,000 and at Hydrogel prepared by reacting with succinimide glutarate at the end. The precursor is administered using a dual chamber applicator that mixes and directs the solution to the site of application.
[0116] All 15 dogs underwent total laminectomy at L2 and L5, after which a 1 cm midline dural incision was made and the incision was sutured. Animals were randomly selected as a control group (n=5 animals; no other treatment before suturing), or DUOFLO Dual-chamber pipette (Hemaedics Inc., Malibu, CA) (n=5 animals) or MICROMYST TM A dual chamber applicator (Confluent Surgical Inc., Waltham, MA) (n=5 animals) administered Formulation 1 at both laminectomy sites. Formulation 1 was observed to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com